Determining folding and binding properties of the C-terminal SH2 domain of SHP2
- PMID: 34605082
- PMCID: PMC8605372
- DOI: 10.1002/pro.4201
Determining folding and binding properties of the C-terminal SH2 domain of SHP2
Abstract
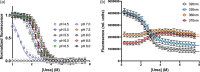
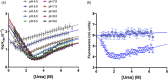
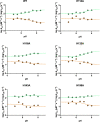
SH2 domains are a class of protein-protein interaction modules with the function to recognize and bind sequences characterized by the presence of a phosphorylated tyrosine. SHP2 is a protein phosphatase involved in the Ras-ERK1/2 signaling pathway that possess two SH2 domains, namely, N-SH2 and C-SH2, that mediate the interaction of SHP2 with various partners and determine the regulation of its catalytic activity. One of the main interactors of the SH2 domains of SHP2 is Gab2, a scaffolding protein with critical role in determining cell differentiation. Despite their key biological role and the importance of a correct native fold to ensure it, the mechanism of binding of SH2 domains with their ligands and the determinants of their stability have been poorly characterized. In this article, we present a comprehensive kinetic study of the folding of the C-SH2 domain and the binding mechanism with a peptide mimicking a region of Gab2. Our data, obtained at different pH and ionic strength conditions and supported by site-directed mutagenesis, highlight the role of electrostatic interactions in the early events of recognition. Interestingly, our results suggest a key role of a highly conserved histidine residue among SH2 family in the interaction with negative charges carried by the phosphotyrosine of Gab2. Moreover, the analysis of the equilibrium and kinetic folding data of C-SH2 describes a complex mechanism implying a change in rate-limiting step at high denaturant concentrations. Our data are discussed under the light of previous works on N-SH2 domain of SHP2 and other SH2 domains.
Keywords: Chevron plot; Gab2; intermediate; kinetics; mutagenesis.
© 2021 The Authors. Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Figures


Similar articles
-
SH2 Domains: Folding, Binding and Therapeutical Approaches.Int J Mol Sci. 2022 Dec 15;23(24):15944. doi: 10.3390/ijms232415944. Int J Mol Sci. 2022. PMID: 36555586 Free PMC article. Review.
-
Folding and Binding Kinetics of the Tandem of SH2 Domains from SHP2.Int J Mol Sci. 2024 Jun 14;25(12):6566. doi: 10.3390/ijms25126566. Int J Mol Sci. 2024. PMID: 38928272 Free PMC article.
-
Mechanism of Folding and Binding of the N-Terminal SH2 Domain from SHP2.J Phys Chem B. 2018 Dec 13;122(49):11108-11114. doi: 10.1021/acs.jpcb.8b05651. Epub 2018 Aug 7. J Phys Chem B. 2018. PMID: 30047735
-
Dissection of the BCR-ABL signaling network using highly specific monobody inhibitors to the SHP2 SH2 domains.Proc Natl Acad Sci U S A. 2013 Sep 10;110(37):14924-9. doi: 10.1073/pnas.1303640110. Epub 2013 Aug 26. Proc Natl Acad Sci U S A. 2013. PMID: 23980151 Free PMC article.
-
Structure and function of Gab2 and its role in cancer (Review).Mol Med Rep. 2015 Sep;12(3):4007-4014. doi: 10.3892/mmr.2015.3951. Epub 2015 Jun 17. Mol Med Rep. 2015. PMID: 26095858 Free PMC article. Review.
Cited by
-
SH2 Domains: Folding, Binding and Therapeutical Approaches.Int J Mol Sci. 2022 Dec 15;23(24):15944. doi: 10.3390/ijms232415944. Int J Mol Sci. 2022. PMID: 36555586 Free PMC article. Review.
-
Folding and Binding Mechanisms of the SH2 Domain from Crkl.Biomolecules. 2022 Jul 22;12(8):1014. doi: 10.3390/biom12081014. Biomolecules. 2022. PMID: 35892324 Free PMC article.
-
Folding and Binding Kinetics of the Tandem of SH2 Domains from SHP2.Int J Mol Sci. 2024 Jun 14;25(12):6566. doi: 10.3390/ijms25126566. Int J Mol Sci. 2024. PMID: 38928272 Free PMC article.
-
Characterization of early and late transition states of the folding pathway of a SH2 domain.Protein Sci. 2022 Jun;31(6):e4332. doi: 10.1002/pro.4332. Protein Sci. 2022. PMID: 35634781 Free PMC article.
-
An intramolecular energetic network regulates ligand recognition in a SH2 domain.Protein Sci. 2023 Aug;32(8):e4729. doi: 10.1002/pro.4729. Protein Sci. 2023. PMID: 37468946 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

