Advanced Maternal Age-associated SIRT1 Deficiency Compromises Trophoblast Epithelial-Mesenchymal Transition through an Increase in Vimentin Acetylation
- PMID: 34605151
- PMCID: PMC8520724
- DOI: 10.1111/acel.13491
Advanced Maternal Age-associated SIRT1 Deficiency Compromises Trophoblast Epithelial-Mesenchymal Transition through an Increase in Vimentin Acetylation
Abstract
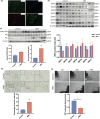
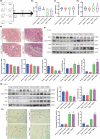
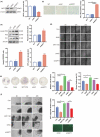
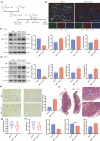
Advanced maternal age (AMA) pregnancies are rapidly increasing and are associated with aberrant trophoblast cell function, poor placentation, and unfavorable pregnancy outcomes, presumably due to premature placental senescence. SIRT1 is an NAD+ -dependent deacetylase with well-known antiaging effects, but its connection with placental senescence is unreported. In this study, human term placentas and first-trimester villi were collected from AMA and normal pregnancies, and a mouse AMA model was established by cross breeding young and aged male and female C57 mice. SIRT1 expression and activity in HTR8/SVneo cells were genetically or pharmacologically manipulated. Trophoblast-specific Sirt1-knockout (KO) mouse placentas were generated by mating Elf5-Cre and Sirt1fl/fl mice. Trophoblast cell mobility was assessed with transwell invasion and wound-healing assays. SIRT1-binding proteins in HTR8/SVneo cells and human placental tissue were identified by mass spectrometry. We identified SIRT1 as the only differentially expressed sirtuin between AMA and normal placentas. It is downregulated in AMA placentas early in the placental life cycle and is barely impacted by paternal age. SIRT1 loss upregulates P53 acetylation and P21 expression and impairs trophoblast invasion and migration. Sirt1-KO mouse placentas exhibit senescence markers and morphological disruption, along with decreased fetal weight. In trophoblasts, SIRT1 interacts with vimentin, regulating its acetylation. In conclusion, SIRT1 promotes trophoblast epithelial-mesenchymal transition (EMT) to enhance invasiveness by modulating vimentin acetylation. AMA placentas are associated with premature senescence during placentation due to SIRT1 loss. Therefore, SIRT1 may be an antiaging therapeutic target for improving placental development and perinatal outcomes in AMA pregnancies.
Keywords: SIRT1; advanced maternal age; epithelial−mesenchymal transition; placenta; senescence; trophoblast.
© 2021 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Arney, K. L. , Bao, S. , Bannister, A. J. , Kouzarides, T. , & Surani, M. A. (2002). Histone methylation defines epigenetic asymmetry in the mouse zygote. The International Journal of Developmental Biology 46(3):317‐20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

