Participation, retention and uptake in a multicentre pre-exposure prophylaxis cohort using online, smartphone-compatible data collection
- PMID: 34605153
- PMCID: PMC9292805
- DOI: 10.1111/hiv.13175
Participation, retention and uptake in a multicentre pre-exposure prophylaxis cohort using online, smartphone-compatible data collection
Abstract
Objectives: The aim of the study was to assess the feasibility of a national pre-exposure prophylaxis (PrEP) programme using smartphone-compatible data collection.
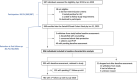
Methods: This was a multicentre cohort study (NCT03893188) enrolling individuals interested in PrEP in Switzerland. All centres participate in the SwissPrEPared programme, which uses smartphone-compatible data collection. Feasibility was assessed after centres had enrolled at least one participant. Participants were HIV-negative individuals presenting for PrEP counselling. Outcomes were participation (number enrolled/number eligible), enrolment rates (number enrolled per month), retention at first follow-up (number with first follow-up/number enrolled), and uptake (proportion attending first visit as scheduled). Participant characteristics were compared between those retained after baseline assessment and those who dropped out.
Results: Between April 2019 and January 2020, 987 individuals were assessed for eligibility, of whom 969 were enrolled (participation: 98.2%). The median enrolment rate was 86 per month [interquartile range (IQR) 52-137]. Retention at first follow-up and uptake were both 80.7% (782/969 and 532/659, respectively). At enrolment, the median age was 40 (IQR 33-47) years, 95% were men who have sex with men, 47% had a university degree, and 75.5% were already taking PrEP. Most reported multiple casual partners (89.2%), previous sexually transmitted infections (74%) and sexualized drug use (73.1%). At baseline, 25.5% tested positive for either syphilis, gonorrhoea or chlamydia. Participants who dropped out were at lower risk of HIV infection than those retained after baseline assessment.
Conclusions: In a national PrEP programme using smartphone-compatible data collection, participation, retention and uptake were high. Participants retained after baseline assessment were at considerable risk of HIV infection. Younger, less educated individuals were underrepresented in the SwissPrEPared cohort.
Keywords: HIV; cohort study; pre-exposure prophylaxis; prevention programme.
© 2021 The Authors. HIV Medicine published by John Wiley & Sons Ltd on behalf of British HIV Association.
Conflict of interest statement
The institution of EB received fees for his participation on advisory boards and travel grants from Gilead Sciences, MSD, ViiV Healthcare, Abbvie, Pfizer, and Sandoz. DLB received honoraria for advisory board participation from Gilead Sciences, MSD and ViiV Healthcare. The institution of KD received research funding unrelated to this publication from Gilead Sciences and sponsorship for attendance at specialist meetings from MSD. MS is an advisory board member of Gilead Sciences, MSD and ViiV Healthcare, and received travel grants from Gilead Sciences. The institution of BS received travel grants from Gilead Sciences. The institution of PT received grants and advisory fees from ViiV Healthcare and Gilead Sciences, outside the submitted work. JSF received research grants unrelated to the submitted work from Merck & Co, Gilead Sciences, and ViiV Healthcare. The institution of BH received research and travel grants from ViiV Healthcare, MSD and Gilead Sciences. The other authors declare no conflicts of interest. Open Access Funding provided by Universitat Zurich.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

