Sex-dependent acrolein sensitivity in mice is associated with differential lung cell, protein, and transcript changes
- PMID: 34605213
- PMCID: PMC8488558
- DOI: 10.14814/phy2.14997
Sex-dependent acrolein sensitivity in mice is associated with differential lung cell, protein, and transcript changes
Abstract
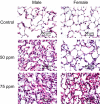
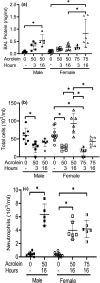
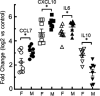
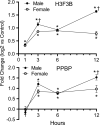
Acrolein is a reactive inhalation hazard. Acrolein's initial interaction, which in itself can be function-altering, is followed by time-dependent cascade of complex cellular and pulmonary responses that dictate the severity of the injury. To investigate the pathophysiological progression of sex-dependent acrolein-induced acute lung injury, C57BL/6J mice were exposed for 30 min to sublethal, but toxic, and lethal acrolein. Male mice were more sensitive than female mice. Acrolein of 50 ppm was sublethal to female but lethal to male mice, and 75 ppm was lethal to female mice. Lethal and sublethal acrolein exposure decreased bronchoalveolar lavage (BAL) total cell number at 3 h after exposure. The cell number decrease was followed by progressive total cell and neutrophil number and protein increases. The BAL total cell number in female mice exposed to a sublethal, but not lethal dose, returned to control levels at 16 h. In contrast, BAL protein content and neutrophil number were higher in mice exposed to lethal compared to sublethal acrolein. RNASeq pathway analysis identified greater increased lung neutrophil, glutathione metabolism, oxidative stress responses, and CCL7 (aka MCP-3), CXCL10 (aka IP-10), and IL6 transcripts in males than females, whereas IL10 increased more in female than male mice. Thus, the IL6:IL10 ratio, an indicator of disease severity, was greater in males than females. Further, H3.3 histone B (H3F3B) and pro-platelet basic protein (PPBP aka CXCL7), transcripts increased in acrolein exposed mouse BAL and plasma at 3 h, while H3F3B protein that is associated with neutrophil extracellular traps formation increased at 12 h. These results suggest that H3F3B and PPBP transcripts increase may contribute to extracellular H3F3B and PPBP proteins increase.
Keywords: NETs; RNAseq; acrolein; acute respiratory distress syndrome.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
None declared.
Figures


Similar articles
-
Analysis of Acrolein Exposure Induced Pulmonary Response in Seven Inbred Mouse Strains and Human Primary Bronchial Epithelial Cells Cultured at Air-Liquid Interface.Biomed Res Int. 2020 Oct 8;2020:3259723. doi: 10.1155/2020/3259723. eCollection 2020. Biomed Res Int. 2020. PMID: 33110918 Free PMC article.
-
Integrative metabolome and transcriptome profiling reveals discordant energetic stress between mouse strains with differential sensitivity to acrolein-induced acute lung injury.Mol Nutr Food Res. 2011 Sep;55(9):1423-34. doi: 10.1002/mnfr.201100291. Epub 2011 Aug 8. Mol Nutr Food Res. 2011. PMID: 21823223 Free PMC article.
-
CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation.J Immunol. 2002 Jan 15;168(2):846-52. doi: 10.4049/jimmunol.168.2.846. J Immunol. 2002. PMID: 11777981
-
Albumin Protects Lung Cells against Acrolein Cytotoxicity and Acrolein-Adducted Albumin Increases Heme Oxygenase 1 Transcripts.Chem Res Toxicol. 2020 Jul 20;33(7):1969-1979. doi: 10.1021/acs.chemrestox.0c00146. Epub 2020 Jun 29. Chem Res Toxicol. 2020. PMID: 32530271
-
Emergency management of chlorine gas exposure - a systematic review.Clin Toxicol (Phila). 2019 Feb;57(2):77-98. doi: 10.1080/15563650.2018.1519193. Epub 2019 Jan 23. Clin Toxicol (Phila). 2019. PMID: 30672349
Cited by
-
Countermeasures against Pulmonary Threat Agents.J Pharmacol Exp Ther. 2024 Jan 17;388(2):560-567. doi: 10.1124/jpet.123.001822. J Pharmacol Exp Ther. 2024. PMID: 37863486 Free PMC article. Review.
-
A Murine Model of Vesicant-Induced Acute Lung Injury.J Pharmacol Exp Ther. 2024 Jan 17;388(2):568-575. doi: 10.1124/jpet.123.001780. J Pharmacol Exp Ther. 2024. PMID: 38050084 Free PMC article.
-
Differential transcriptomic alterations in nasal versus lung tissue of acrolein-exposed rats.Front Toxicol. 2023 Nov 27;5:1280230. doi: 10.3389/ftox.2023.1280230. eCollection 2023. Front Toxicol. 2023. PMID: 38090360 Free PMC article.
-
Multifaceted Roles of Chemokine C-X-C Motif Ligand 7 in Inflammatory Diseases and Cancer.Front Pharmacol. 2022 Jun 28;13:914730. doi: 10.3389/fphar.2022.914730. eCollection 2022. Front Pharmacol. 2022. PMID: 35837284 Free PMC article. Review.
References
-
- Arumugam, S., Girish Subbiah, K., Kemparaju, K., & Thirunavukkarasu, C. (2018). Neutrophil extracellular traps in acrolein promoted hepatic ischemia reperfusion injury: Therapeutic potential of NOX2 and p38MAPK inhibitors. Journal of Cellular Physiology, 233(4), 3244–3261. 10.1002/jcp.26167. - DOI - PubMed
-
- Bdeir, K., Gollomp, K., Stasiak, M., Mei, J., Papiewska‐Pajak, I., Zhao, G., Worthen, G. S., Cines, D. B., Poncz, M., & Kowalska, M. A. (2017). Platelet‐specific chemokines contribute to the pathogenesis of acute lung injury. American Journal of Respiratory Cell and Molecular Biology, 56(2), 261–270. 10.1165/rcmb.2015-0245OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

