Rapid and sustained B-cell depletion with subcutaneous ofatumumab in relapsing multiple sclerosis: APLIOS, a randomized phase-2 study
- PMID: 34605319
- PMCID: PMC9024029
- DOI: 10.1177/13524585211044479
Rapid and sustained B-cell depletion with subcutaneous ofatumumab in relapsing multiple sclerosis: APLIOS, a randomized phase-2 study
Abstract
Background: Ofatumumab, the first fully human anti-CD20 monoclonal antibody, is approved in several countries for relapsing multiple sclerosis (RMS).
Objective: To demonstrate the bioequivalence of ofatumumab administered by an autoinjector versus a pre-filled syringe (PFS) and to explore the effect of ofatumumab on B-cell depletion.
Methods: APLIOS (NCT03560739) is a 12-week, open-label, parallel-group, phase-2 study in patients with RMS receiving subcutaneous ofatumumab 20 mg every 4 weeks (q4w) (from Week 4, after initial doses on Days 1, 7, and 14). Patients were randomized 10:10:1:1 to autoinjector or PFS in the abdomen, or autoinjector or PFS in the thigh, respectively. Bioequivalence was determined by area under the curve (AUCτ) and maximum plasma concentration (Cmax) for Weeks 8-12. B-cell depletion and safety/tolerability were assessed.
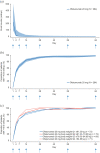
Results: A total of 256 patients contributed to the bioequivalence analyses (autoinjector-abdomen, n = 128; PFS-abdomen, n = 128). Abdominal ofatumumab pharmacokinetic exposure was bioequivalent for autoinjector and PFS (geometric mean AUCτ, 487.7 vs 474.1 h × µg/mL (ratio 1.03); Cmax, 1.409 vs 1.409 µg/mL (ratio 1.00)). B-cell counts (median cells/µL) depleted rapidly in all groups from 214.0 (baseline) to 2.0 (Day 14). Ofatumumab was well tolerated.
Conclusion: Ofatumumab 20 mg q4w self-administered subcutaneously via autoinjector is bioequivalent to PFS administration and provides rapid B-cell depletion.
Keywords: Ofatumumab; autoinjector pen; bioequivalence; multiple sclerosis; pharmacokinetics; pre-filled syringe.
Conflict of interest statement
Figures


References
-
- Hauser SL, Bar-Or A, Cohen JA, et al.. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med 2020; 383: 546–557. - PubMed
-
- Hauser SL, Bar-Or A, Comi G, et al.. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376: 221–234. - PubMed
-
- Hauser SL, Waubant E, Arnold DL, et al.. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008; 358: 676–688. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

