Crystal structures of human coronavirus NL63 main protease at different pH values
- PMID: 34605439
- PMCID: PMC8488857
- DOI: 10.1107/S2053230X21009523
Crystal structures of human coronavirus NL63 main protease at different pH values
Abstract
Human coronavirus NL63 (HCoV-NL63), which belongs to the genus Alphacoronavirus, mainly infects children and the immunocompromized and is responsible for a series of clinical manifestations, including cough, fever, rhinorrhoea, bronchiolitis and croup. HCoV-NL63, which was first isolated from a seven-month-old child in 2004, has led to infections worldwide and accounts for 10% of all respiratory illnesses caused by etiological agents. However, effective antivirals against HCoV-NL63 infection are currently unavailable. The HCoV-NL63 main protease (Mpro), also called 3C-like protease (3CLpro), plays a vital role in mediating viral replication and transcription by catalyzing the cleavage of replicase polyproteins (pp1a and pp1ab) into functional subunits. Moreover, Mpro is highly conserved among all coronaviruses, thus making it a prominent drug target for antiviral therapy. Here, four crystal structures of HCoV-NL63 Mpro in the apo form at different pH values are reported at resolutions of up to 1.78 Å. Comparison with Mpro from other human betacoronaviruses such as SARS-CoV-2 and SARS-CoV reveals common and distinct structural features in different genera and extends knowledge of the diversity, function and evolution of coronaviruses.
Keywords: apo structure; coronaviruses; crystallization; human coronavirus NL63; main protease.
Figures
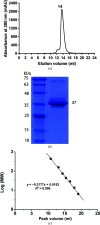

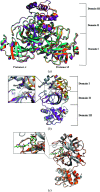


References
-
- Anand, K., Ziebuhr, J., Wadhwani, P., Mesters, J. R. & Hilgenfeld, R. (2003). Science, 300, 1763–1767. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

