Spotlight on eltrombopag in pediatric ITP in China: a long-term observational study in real-world practice
- PMID: 34605871
- PMCID: PMC8679667
- DOI: 10.1182/bloodadvances.2020004110
Spotlight on eltrombopag in pediatric ITP in China: a long-term observational study in real-world practice
Abstract
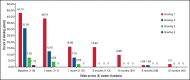
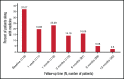
Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder with isolated thrombocytopenia and risk of hemorrhage. Treatment with eltrombopag increases and maintains hemostatic platelet counts; however, to date, long-term data are lacking on the outcome of children with ITP who are treated with eltrombopag. This prospective, observational, longitudinal cohort study evaluated the efficacy and safety of eltrombopag in pediatric patients with persistent or chronic ITP. For the 116 pediatric patients enrolled, duration of eltrombopag treatment was at least 3 months. Median effective dose was 25 mg/day, 50 mg/day, and 50 mg/day, respectively, for children age 5 years or younger, 6 to 11 years, or 12 years or older. In all, 89 patients (76.7%) achieved overall response, 53 (45.7%) achieved complete response, and 36 (31.0%) achieved response. Median platelet counts increased by week 1 and were sustained throughout the treatment period. During treatment with eltrombopag, the proportion of patients with grade 1 to 4 bleeding symptoms decreased from 83.61% at baseline to 9.88% at 6 months when only grade 1 was reported. Forty-three patients (37.1%) reported using concomitant medications at study entry, which was reduced to 1 patient (2.5%) who needed concomitant medications at 12 months. All adverse events were grade 1 or 2 according to Common Terminology Criteria for Adverse Events. No serious adverse events, cataracts, malignancies, or thromboses were reported during the study. Long-term treatment with eltrombopag was generally safe, well tolerated, and effective in maintaining platelet counts and reducing bleeding in most pediatric patients with persistent or chronic ITP. Combined with future studies, these findings will help establish how eltrombopag should best be used in the management of pediatric patients with East Asian ancestry.
© 2021 by The American Society of Hematology.
Figures
References
-
- Liu XG, Bai XC, Chen FP, et al. Chinese guidelines for treatment of adult primary immune thrombocytopenia. Int J Hematol. 2018;107(6):615-623. - PubMed
-
- Singh G, Bansal D, Wright NAM.. Immune thrombocytopenia in children: Consensus and controversies. Indian J Pediatr. 2020;87(2):150-157. - PubMed
-
- Kühne T, Buchanan GR, Zimmerman S, et al. Intercontinental Childhood ITP Study Group. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 2003;143(5):605-608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical