Personalized brain models identify neurotransmitter receptor changes in Alzheimer's disease
- PMID: 34605898
- PMCID: PMC9423713
- DOI: 10.1093/brain/awab375
Personalized brain models identify neurotransmitter receptor changes in Alzheimer's disease
Abstract
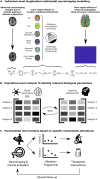
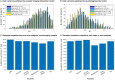
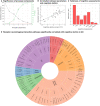
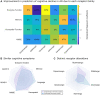
Alzheimer's disease involves many neurobiological alterations from molecular to macroscopic spatial scales, but we currently lack integrative, mechanistic brain models characterizing how factors across different biological scales interact to cause clinical deterioration in a way that is subject-specific or personalized. As important signalling molecules and mediators of many neurobiological interactions, neurotransmitter receptors are promising candidates for identifying molecular mechanisms and drug targets in Alzheimer's disease. We present a neurotransmitter receptor-enriched multifactorial brain model, which integrates spatial distribution patterns of 15 neurotransmitter receptors from post-mortem autoradiography with multiple in vivo neuroimaging modalities (tau, amyloid-β and glucose PET, and structural, functional and arterial spin labelling MRI) in a personalized, generative, whole-brain formulation. In a heterogeneous aged population (n = 423, ADNI data), models with personalized receptor-neuroimaging interactions showed a significant improvement over neuroimaging-only models, explaining about 70% (±20%) of the variance in longitudinal changes to the six neuroimaging modalities. In Alzheimer's disease patients (n = 25, ADNI data), receptor-imaging interactions explained up to 39.7% (P < 0.003, family-wise error-rate-corrected) of inter-individual variability in cognitive deterioration, via an axis primarily affecting executive function. Notably, based on their contribution to the clinical severity in Alzheimer's disease, we found significant functional alterations to glutamatergic interactions affecting tau accumulation and neural activity dysfunction and GABAergic interactions concurrently affecting neural activity dysfunction, amyloid and tau distributions, as well as significant cholinergic receptor effects on tau accumulation. Overall, GABAergic alterations had the largest effect on cognitive impairment (particularly executive function) in our Alzheimer's disease cohort (n = 25). Furthermore, we demonstrate the clinical applicability of this approach by characterizing subjects based on individualized 'fingerprints' of receptor alterations. This study introduces the first robust, data-driven framework for integrating several neurotransmitter receptors, multimodal neuroimaging and clinical data in a flexible and interpretable brain model. It enables further understanding of the mechanistic neuropathological basis of neurodegenerative progression and heterogeneity, and constitutes a promising step towards implementing personalized, neurotransmitter-based treatments.
Keywords: Alzheimer’s disease; multimodal neuroimaging; neurotransmitter receptors; personalized medicine; whole-brain computational model.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


Similar articles
-
Combining tau-PET and fMRI meta-analyses for patient-centered prediction of cognitive decline in Alzheimer's disease.Alzheimers Res Ther. 2022 Nov 7;14(1):166. doi: 10.1186/s13195-022-01105-5. Alzheimers Res Ther. 2022. PMID: 36345046 Free PMC article.
-
Connectomics and neurotransmitter receptor profile explain regional tau pathology in Alzheimer's disease.Cereb Cortex. 2025 Mar 6;35(3):bhaf053. doi: 10.1093/cercor/bhaf053. Cereb Cortex. 2025. PMID: 40083151
-
Molecular properties underlying regional vulnerability to Alzheimer's disease pathology.Brain. 2018 Sep 1;141(9):2755-2771. doi: 10.1093/brain/awy189. Brain. 2018. PMID: 30016411 Free PMC article.
-
Update on neuroimaging in Alzheimer's disease.Curr Opin Neurol. 2021 Aug 1;34(4):525-531. doi: 10.1097/WCO.0000000000000947. Curr Opin Neurol. 2021. PMID: 33928929 Free PMC article. Review.
-
Molecular imaging of dementia.Psychogeriatrics. 2012 Jun;12(2):106-14. doi: 10.1111/j.1479-8301.2012.00409.x. Psychogeriatrics. 2012. PMID: 22712644 Review.
Cited by
-
Patient-centered brain transcriptomic and multimodal imaging determinants of clinical progression, physical activity, and treatment needs in Parkinson's disease.NPJ Parkinsons Dis. 2025 Feb 15;11(1):29. doi: 10.1038/s41531-025-00878-4. NPJ Parkinsons Dis. 2025. PMID: 39952947 Free PMC article.
-
Translating the Post-Mortem Brain Multi-Omics Molecular Taxonomy of Alzheimer's Dementia to Living Humans.bioRxiv [Preprint]. 2025 Mar 24:2025.03.20.644323. doi: 10.1101/2025.03.20.644323. bioRxiv. 2025. PMID: 40196602 Free PMC article. Preprint.
-
Macroscale connectome topographical structure reveals the biomechanisms of brain dysfunction in Alzheimer's disease.Sci Adv. 2024 Oct 11;10(41):eado8837. doi: 10.1126/sciadv.ado8837. Epub 2024 Oct 11. Sci Adv. 2024. PMID: 39392880 Free PMC article.
-
Brain connectomics: time for a molecular imaging perspective?Trends Cogn Sci. 2023 Apr;27(4):353-366. doi: 10.1016/j.tics.2022.11.015. Epub 2023 Jan 6. Trends Cogn Sci. 2023. PMID: 36621368 Free PMC article. Review.
-
Revealing the combined roles of Aβ and tau in Alzheimer's disease via a pathophysiological activity decoder.bioRxiv [Preprint]. 2023 Jul 20:2023.02.21.529377. doi: 10.1101/2023.02.21.529377. bioRxiv. 2023. PMID: 37502947 Free PMC article. Preprint.
References
-
- Francis PT, Ramírez MJ, Lai MK.. Neurochemical basis for symptomatic treatment of Alzheimer’s disease. Neuropharmacology. 2010;59(4-5):221–229. - PubMed
-
- Iturria-Medina Y, Carbonell FM, Evans AC.. Multimodal imaging-based therapeutic fingerprints for optimizing personalized interventions: Application to neurodegeneration. NeuroImage. 2018;179:40–50. - PubMed
-
- Kosik KS. Personalized medicine for effective Alzheimer disease treatment. JAMA Neurol. 2015;72(5):497–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

