Safety and tolerability of non-neutralizing adrenomedullin antibody adrecizumab (HAM8101) in septic shock patients: the AdrenOSS-2 phase 2a biomarker-guided trial
- PMID: 34605947
- PMCID: PMC8487806
- DOI: 10.1007/s00134-021-06537-5
Safety and tolerability of non-neutralizing adrenomedullin antibody adrecizumab (HAM8101) in septic shock patients: the AdrenOSS-2 phase 2a biomarker-guided trial
Abstract
Purpose: Investigate safety and tolerability of adrecizumab, a humanized monoclonal adrenomedullin antibody, in septic shock patients with high adrenomedullin.
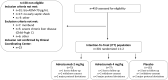
Methods: Phase-2a, double-blind, randomized, placebo-controlled biomarker-guided trial with a single infusion of adrecizumab (2 or 4 mg/kg b.w.) compared to placebo. Patients with adrenomedullin above 70 pg/mL, < 12 h of vasopressor start for septic shock were eligible. Randomization was 1:1:2. Primary safety (90-day mortality, treatment emergent adverse events (TEAE)) and tolerability (drug interruption, hemodynamics) endpoints were recorded. Efficacy endpoints included the Sepsis Support Index (SSI, reflecting ventilator- and shock-free days alive), change in Sequential-related Organ Failure Assessment (SOFA) and 28-day mortality.
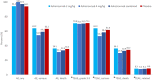
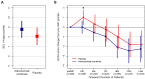
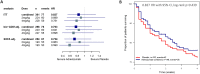
Results: 301 patients were enrolled (median time of 8.5 h after vasopressor start). Adrecizumab was well tolerated (one interruption, no hemodynamic alteration) with no differences in frequency and severity in TEAEs between treatment arms (TEAE of grade 3 or higher: 70.5% in the adrecizumab group and 71.1% in the placebo group) nor in 90-day mortality. Difference in change in SSI between adrecizumab and placebo was 0.72 (CI -1.93-0.49, p = 0.24). Among various secondary endpoints, delta SOFA score (defined as maximum versus minimum SOFA) was more pronounced in the adrecizumab combined group compared to placebo [difference at 0.76 (95% CI 0.18-1.35); p = 0.007]. 28-day mortality in the adrecizumab group was 23.9% and 27.7% in placebo with a hazard ratio of 0.84 (95% confidence interval 0.53-1.31, log-rank p = 0.44).
Conclusions: Overall, we successfully completed a randomized trial evaluating selecting patients for enrolment who had a disease-related biomarker. There were no overt signals of harm with using two doses of the adrenomedullin antibody adrecizumab; however, further randomized controlled trials are required to confirm efficacy and safety of this agent in septic shock patients.
Trial registration: ClinicalTrials.gov NCT03085758.
Keywords: Adrecizumab (HAM8101); Adrenomedullin; Endothelial function; Enibarcimab; Septic shock.
© 2021. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
P-F L received fees as a coordinator of the CCC. PP reports travel and consultancy reimbursement from Adrenomed, SphingoTec, 4TEEN4, AM-Pharma, Baxter, EBI. XW was part of the Clinical Coordinating Center assessing patient’s eligibility. No other conflict of interest. FM has no conflict of interest. TD received fees as a coordinator of the CCC. BF reports personal fees outside the submitted work from Inotrem, Biomérieux, AM-Pharma, Takeda, Enlivex, Aridis, GSK, Asahi-Kasai. JBL reported receiving consultation fees from Asahi Kasei America Corporation outside the submitted work. AB Consultancy fees from GSK and Danone Nutricia. DC has no conflict of interest. OH has no conflict of interest. JCL has no conflict of interest to declare. BC served as a member of an advisory board for Roche Diagnostics. JP has no conflict of interest with the present work. MB holds shares of SmartDyeLivery GmbH, Jena. He has received funding for scientific advisory boards, travel and speaker honoraria by T2 Biosystems, Inc., La Jolla Pharmaceutical Company, SNIPR BIOME Denmark, CytoSorbents GmbH, Thermo Fisher Scientific (B·R·A·H·M·S GmbH), Roche Diagnostics International Ltd., Transgene S.A. and SphingoTec GmbH. TG has non conflict of interest with the present work. MK reports grant support from Adrenomed and Vifor, and honoraria from Adrenomed, SphingoTec, Vifor, Amgen, 4TEEN4, Astra-Zeneca, and Sanofi. AM reports personal fees from Orion, Sanofi, Adrenomed, Epygon and Fire 1 and grants and personal fees from 4TEEN4, Abbott, Roche and SphingoTec.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

