Chiral Photocatalyst Structures in Asymmetric Photochemical Synthesis
- PMID: 34606251
- PMCID: PMC8792375
- DOI: 10.1021/acs.chemrev.1c00467
Chiral Photocatalyst Structures in Asymmetric Photochemical Synthesis
Abstract
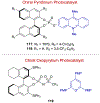
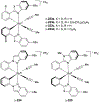
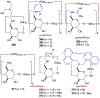
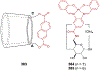
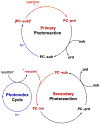
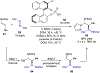
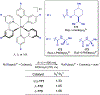
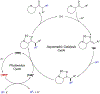
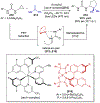
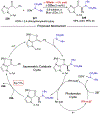
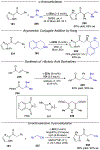
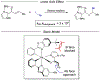
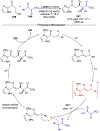
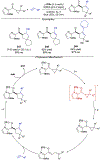
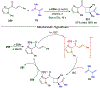
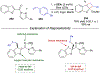
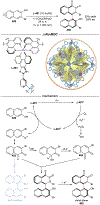
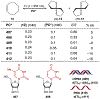
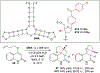
Asymmetric catalysis is a major theme of research in contemporary synthetic organic chemistry. The discovery of general strategies for highly enantioselective photochemical reactions, however, has been a relatively recent development, and the variety of photoreactions that can be conducted in a stereocontrolled manner is consequently somewhat limited. Asymmetric photocatalysis is complicated by the short lifetimes and high reactivities characteristic of photogenerated reactive intermediates; the design of catalyst architectures that can provide effective enantiodifferentiating environments for these intermediates while minimizing the participation of uncontrolled racemic background processes has proven to be a key challenge for progress in this field. This review provides a summary of the chiral catalyst structures that have been studied for solution-phase asymmetric photochemistry, including chiral organic sensitizers, inorganic chromophores, and soluble macromolecules. While some of these photocatalysts are derived from privileged catalyst structures that are effective for both ground-state and photochemical transformations, others are structural designs unique to photocatalysis and offer insight into the logic required for highly effective stereocontrolled photocatalysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

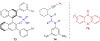



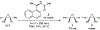






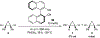

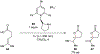
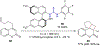
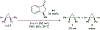

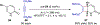
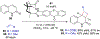
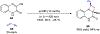

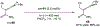
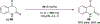
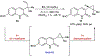

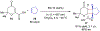
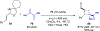

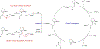
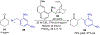







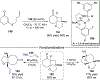
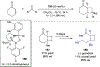
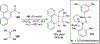
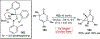





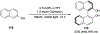



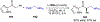
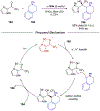
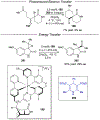
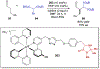

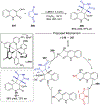






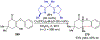
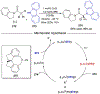
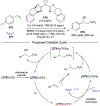


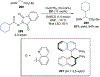
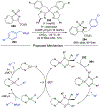




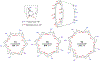
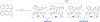
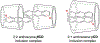


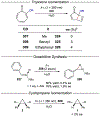
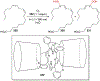

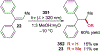
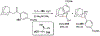
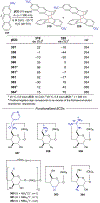



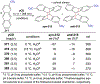
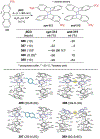
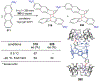



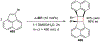




References
-
- Sharpless KB Searching for New Reactivity (Nobel Lecture). Angew. Chem. Int. Ed 2002, 41, 2024–2032. - PubMed
-
- Noyori R Asymmetric Catalysis: Science and Opportunities (Nobel Lecture 2001). Adv. Synth. Catal 2003, 345, 15–32. - PubMed
-
- Knowles WS Asymmetric Hydrogenations (Nobel Lecture 2001). Adv. Synth. Catal 2003, 345, 3–13.
-
- Ciamician G The Photochemistry of the Future. Science 1912, 36, 385–394. - PubMed
-
- Roth HD The Beginnings of Organic Photochemistry. Angew. Chem. Int. Ed 1989, 28, 1193–1207.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

