Understanding Halide Counterion Effects in Enantioselective Ruthenium-Catalyzed Carbonyl (α-Aryl)allylation: Alkynes as Latent Allenes and Trifluoroethanol-Enhanced Turnover in The Conversion of Ethanol to Higher Alcohols via Hydrogen Auto-transfer
- PMID: 34606271
- PMCID: PMC8749865
- DOI: 10.1021/jacs.1c07857
Understanding Halide Counterion Effects in Enantioselective Ruthenium-Catalyzed Carbonyl (α-Aryl)allylation: Alkynes as Latent Allenes and Trifluoroethanol-Enhanced Turnover in The Conversion of Ethanol to Higher Alcohols via Hydrogen Auto-transfer
Abstract
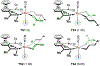
Crystallographic characterization of RuX(CO)(η3-C3H5)(JOSIPHOS), where X = Cl, Br, or I, reveals a halide-dependent diastereomeric preference that defines metal-centered stereogenicity and, therefrom, the enantioselectivity of C-C coupling in ruthenium-catalyzed anti-diastereo- and enantioselective C-C couplings of primary alcohols with 1-aryl-1-propynes to form products of carbonyl anti-(α-aryl)allylation. Computational studies reveal that a non-classical hydrogen bond between iodide and the aldehyde formyl CH bond stabilizes the favored transition state for carbonyl addition. An improved catalytic system enabling previously unattainable transformations was developed that employs an iodide-containing precatalyst, RuI(CO)3(η3-C3H5), in combination with trifluoroethanol, as illustrated by the first enantioselective ruthenium-catalyzed C-C couplings of ethanol to form higher alcohols.
Figures

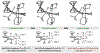

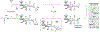
References
-
- For a recent review on metal-catalyzed C-C coupling of feedstocks, see: Doerksen RS; Meyer CC; Krische MJ Feedstock Reagents in Metal-Catalyzed Carbonyl Reductive Coupling: Minimizing Preactivation for Efficiency in Target-Oriented Synthesis. Angew. Chem., Int. Ed 2019, 58, 14055–14064. - PMC - PubMed
-
- For selected reviews on metal-catalyzed carbonyl addition via hydrogen auto-transfer, see: Ketcham JM; Shin I; Montgomery TP; Krische MJ Catalytic Enantioselective C-H Functionalization of Alcohols by Redox-Triggered Carbonyl Addition: Borrowing Hydrogen, Returning Carbon. Angew. Chem. Int. Ed 2014, 53, 9142–9150. - PMC - PubMed
- Kim SW; Zhang W; Krische MJ Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol-Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier. Acc. Chem. Res 2017, 50, 2371–2380. - PMC - PubMed
- Santana CG; Krische MJ From Hydrogenation to Transfer Hydrogenation to Hydrogen Auto-Transfer in Enantioselective Metal-Catalyzed Carbonyl Reductive Coupling: Past, Present and Future. ACS Catal. 2021, 11, 5572–5585. - PMC - PubMed
-
- For selected reviews on metal-catalyzed hydroxyl substitution via hydrogen auto-transfer, see: Hamid MHSA; Slatford PA; Williams JMJ Borrowing Hydrogen in The Activation of Alcohols. Adv. Synth. Catal 2007, 349, 1555–1575.
- Guillena G; Ramón DJ; Yus M Alcohols as Electrophiles in C-C Bond-Forming Reactions: The Hydrogen Autotransfer Process. Angew. Chem. Int. Ed 2007, 46, 2358–2364.
- Dobereiner GE; Crabtree RH Dehydrogenation as a Substrate-Activating Strategy in Homogeneous Transition Metal Catalysis. Chem. Rev 2010, 110, 681–703.
- Bähn S; Imm S; Neubert L; Zhang M; Neumann H; Beller M The Catalytic Amination of Alcohols. ChemCatChem 2011, 3, 1853–1864.
- Yang Q; Wang Q; Yu Z Substitution of Alcohols by N-Nucleophiles via Transition Metal-Catalyzed Dehydrogenation. Chem. Soc. Rev 2015, 44, 2305–2329.
- Aitchison H; Wingad RL; Wass DF Homogeneous Ethanol to Butanol Catalysis - Guerbet Renewed. ACS Catal. 2016, 6, 7125–7132.
- Quintard A; Rodriguez J Catalytic Enantioselective OFF ↔ ON Activation Processes Initiated by Hydrogen Transfer: Concepts and Challenges. Chem. Commun 2016, 52, 10456–10473.
- Reed-Berendt BG; Polidano K; Morrill LC Recent Advances in Homogeneous Borrowing Hydrogen Catalysis Using Earth-Abundant First Row Transition Metals. Org. Biomol. Chem 2019, 17, 1595–1607.
- Kwok T; Hoff O; Armstrong RJ; Donohoe TJ Control of Absolute Stereochemistry in Transition-Metal-Catalysed Hydrogen-Borrowing Reactions. Chem. Eur. J 2020, 26, 12912–12926.
- Reed-Berendt BG; Latham DE; Dambatta MB; Morrill LC Borrowing Hydrogen for Organic Synthesis. ACS Cent. Sci 2021, 7, 570–585.
-
- For recent reports on the use of ethanol in Guerbet-type reactions, see: Mazzoni R; Cesari C; Zanotti V; Lucarelli C; Tabanelli T; Puzzo F; Passarini F; Neri E; Marani G; Prati R; Vigano F; Conversano A; Cavani F Catalytic Biorefining of Ethanol from Wine Waste to Butanol and Higher Alcohols: Modeling the Life Cycle Assessment and Process Design. ACS Sus. Chem. Eng 2019, 7, 224–237.
- Gawali SS; Pandia BK; Pal S; Gunanathan C Manganese(I)-Catalyzed Cross-Coupling of Ketones and Secondary Alcohols with Primary Alcohols. ACS Omega 2019, 4, 10741–10754.
- Kobayashi M; Itoh S; Yoshimura K; Tsukamoto Y; Obora Y Iridium Complex-Catalyzed C2-Extension of Primary Alcohols with Ethanol via a Hydrogen Autotransfer Reaction. J. Org. Chem 2020, 85, 11952–11958.
-
- For selected examples of enantioselective Guerbet-type reactions, see: Onodera G; Nishibayashi Y; Uemura S Ir- and Ru-Catalyzed Sequential Reactions: Asymmetric α-Alkylative Reduction of Ketones with Alcohols. Angew. Chem. Int. Ed 2006, 45, 3819–3822. - PubMed
- Armstrong RJ; Akhtar WM; Young TA; Duarte F; Donohoe TJ Catalytic Asymmetric Synthesis of Cyclohexanes by Hydrogen Borrowing Annulations. Angew. Chem. Int. Ed 2019, 58, 12558–12562. - PubMed
- Ng TW; Liao G; Lau KK; Pan H-J; Zhao Y Room-Temperature Guerbet Reaction with Unprecedented Catalytic Efficiency and Enantioselectivity. Angew. Chem. Int. Ed 2020, 59, 11384–11389. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

