Emerging Roles of DLK1 in the Stem Cell Niche and Cancer Stemness
- PMID: 34606325
- PMCID: PMC8721543
- DOI: 10.1369/00221554211048951
Emerging Roles of DLK1 in the Stem Cell Niche and Cancer Stemness
Abstract
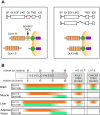
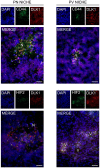
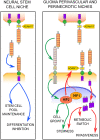
DLK1 is a maternally imprinted, paternally expressed gene coding for the transmembrane protein Delta-like homologue 1 (DLK1), a non-canonical NOTCH ligand with well-described roles during development, and tumor-supportive functions in several aggressive cancer forms. Here, we review the many functions of DLK1 as a regulator of stem cell pools and tissue differentiation in tissues such as brain, muscle, and liver. Furthermore, we review recent evidence supporting roles for DLK1 in the maintenance of aggressive stem cell characteristics of tumor cells, specifically focusing on central nervous system tumors, neuroblastoma, and hepatocellular carcinoma. We discuss NOTCH -dependent as well as NOTCH-independent functions of DLK1, and focus particularly on the complex pattern of DLK1 expression and cleavage that is finely regulated from a spatial and temporal perspective. Progress in recent years suggest differential functions of extracellular, soluble DLK1 as a paracrine stem cell niche-secreted factor, and has revealed a role for the intracellular domain of DLK1 in cell signaling and tumor stemness. A better understanding of DLK1 regulation and signaling may enable therapeutic targeting of cancer stemness by interfering with DLK1 release and/or intracellular signaling.
Keywords: DLK1; angiogenesis; cancer; glioblastoma; stem cell; stemness; tissue differentiation; tumor heterogeneity; tumor immune infiltrate; tumor microenvironment.
Conflict of interest statement
Figures
References
-
- Ferrón SR, Charalambous M, Radford E, McEwen K, Wildner H, Hind E, Morante-Redolat JM, Laborda J, Guillemot F, Bauer SR, Fariñas I, Ferguson-Smith AC. Postnatal loss of DLK1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 2011;475(7356):381–5. doi:10.1038/nature10229. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

