Testing approaches to sharing trial results with participants: The Show RESPECT cluster randomised, factorial, mixed methods trial
- PMID: 34606495
- PMCID: PMC8523080
- DOI: 10.1371/journal.pmed.1003798
Testing approaches to sharing trial results with participants: The Show RESPECT cluster randomised, factorial, mixed methods trial
Abstract
Background: Sharing trial results with participants is an ethical imperative but often does not happen. We tested an Enhanced Webpage versus a Basic Webpage, Mailed Printed Summary versus no Mailed Printed Summary, and Email List Invitation versus no Email List Invitation to see which approach resulted in the highest patient satisfaction with how the results were communicated.
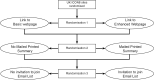
Methods and findings: We carried out a cluster randomised, 2 by 2 by 2 factorial, nonblinded study within a trial, with semistructured qualitative interviews with some patients (ISRCTN96189403). Each cluster was a UK hospital participating in the ICON8 ovarian cancer trial. Interventions were shared with 384 ICON8 participants who were alive and considered well enough to be contacted, at 43 hospitals. Hospitals were allocated to share results with participants through one of the 8 intervention combinations based on random permutation within blocks of 8, stratified by number of participants. All interventions contained a written plain English summary of the results. The Enhanced Webpage also contained a short video. Both the Enhanced Webpage and Email contained links to further information and support. The Mailed Printed Summary was opt-out. Follow-up questionnaires were sent 1 month after patients had been offered the interventions. Patients' reported satisfaction was measured using a 5-point scale, analysed by ordinal logistic regression estimating main effects for all 3 interventions, with random effects for site, restricted to those who reported receiving the results and assuming no interaction. Data collection took place in 2018 to 2019. Questionnaires were sent to 275/384 randomly selected participants and returned by 180: 90/142 allocated Basic Webpage, 90/133 Enhanced Webpage; 91/141 no Mailed Printed Summary, 89/134 Mailed Printed Summary; 82/129 no Email List Invitation, 98/146 Email List Invitation. Only 3 patients opted out of receiving the Mailed Printed Summary; no patients signed up to the email list. Patients' satisfaction was greater at sites allocated the Mailed Printed Summary, where 65/81 (80%) were quite or very satisfied compared to sites with no Mailed Printed Summary 39/64 (61%), ordinal odds ratio (OR) = 3.15 (1.66 to 5.98, p < 0.001). We found no effect on patient satisfaction from the Enhanced Webpage, OR = 1.47 (0.78 to 2.76, p = 0.235) or Email List Invitation, OR = 1.38 (0.72 to 2.63, p = 0.327). Interviewees described the results as interesting, important, and disappointing (the ICON8 trial found no benefit). Finding out the results made some feel their trial participation had been more worthwhile. Regardless of allocated group, patients who received results generally reported that the information was easy to understand and find, were glad and did not regret finding out the results. The main limitation of our study is the 65% response rate.
Conclusions: Nearly all respondents wanted to know the results and were glad to receive them. Adding an opt-out Mailed Printed Summary alongside a webpage yielded the highest reported satisfaction. This study provides evidence on how to share results with other similar trial populations. Further research is needed to look at different results scenarios and patient populations.
Trial registration: ISRCTN: ISRCTN96189403.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: AS, ECJ, CP, CT, CS, AM, AJC, NJH, CDM, KG, TI, BEB and WJC have nothing to declare. MRS reports grants and non-financial support from Astellas, grants from Clovis, grants and non-financial support from Janssen, grants and non-financial support from Novartis, grants and non-financial support from Pfizer, grants and non-financial support from Sanofi, personal fees from Lilly Oncology, personal fees from Janssen, outside the submitted work.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

