Species diversity and distribution of schistosome intermediate snail hosts in The Gambia
- PMID: 34606509
- PMCID: PMC8516291
- DOI: 10.1371/journal.pntd.0009823
Species diversity and distribution of schistosome intermediate snail hosts in The Gambia
Abstract
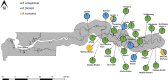
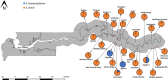
There is a need for recent information on intermediate snail hosts of schistosomes in The Gambia; the previous studies were conducted over three decades ago. This study assessed the incidence, species diversity, distribution and infection status of schistosome intermediate snail hosts in the country. Malacological surveys were conducted in all 5 regions of The Gambia: Central River Region (CRR), Upper River Region (URR), Western Region (WR), Lower River Region (LRR) and North Bank Region (NBR). Sampling of snails was undertaken at 114 sites that included permanent water bodies such as streams (bolongs), rice fields, irrigation canals and swamps; and temporal (seasonal) laterite pools. Ecological and physicochemical factors of sites were recorded. Snails were identified morphologically and screened for schistosome infections using molecular techniques. Freshwater snails were found at more than 50% (60/114) of sites sampled. While three species of Bulinus were collected, no Biomphalaria snails were found in any of the sites sampled. Of the total 2877 Bulinus snails collected, 75.9% were identified as Bulinus senegalensis, 20.9% as Bulinus forskalii and 3.2% as Bulinus truncatus. Seasonal pools produced the largest number of snails, and CRR was the region with the largest number of snails. Bulinus senegalensis was found more in seasonal pools as opposed to permanent sites, where B. forskalii and B. truncatus were observed to thrive. Bulinus snails were more common in seasonal sites where aquatic vegetation was present. In permanent sites, the abundance of snails increased with increase in water temperature and decrease in water pH. Bulinus senegalensis was found infected with both S. haematobium and S. bovis, while B. forskalii and B. truncatus had only S. bovis infection. While the human parasite S. haematobium was restricted to just four sites, the livestock parasite S. bovis had a much more widespread geographical distribution across both CRR and URR. This new information on the distribution of intermediate snail hosts of schistosomes in The Gambia will be vital for the national schistosomiasis control initiative.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Interactions between Schistosoma haematobium group species and their Bulinus spp. intermediate hosts along the Niger River Valley.Parasit Vectors. 2020 May 24;13(1):268. doi: 10.1186/s13071-020-04136-9. Parasit Vectors. 2020. PMID: 32448268 Free PMC article.
-
Freshwater snails of biomedical importance in the Niger River Valley: evidence of temporal and spatial patterns in abundance, distribution and infection with Schistosoma spp.Parasit Vectors. 2019 Oct 22;12(1):498. doi: 10.1186/s13071-019-3745-8. Parasit Vectors. 2019. PMID: 31640811 Free PMC article.
-
Molecular characterization and distribution of Schistosoma cercariae collected from naturally infected bulinid snails in northern and central Côte d'Ivoire.Parasit Vectors. 2019 Mar 19;12(1):117. doi: 10.1186/s13071-019-3381-3. Parasit Vectors. 2019. PMID: 30890180 Free PMC article.
-
Studies on transmission and schistosome interactions in Senegal, Mali and Zambia.Trop Geogr Med. 1994;46(4 Spec No):220-6. Trop Geogr Med. 1994. PMID: 7825224 Review.
-
Interactions between intermediate snail hosts of the genus Bulinus and schistosomes of the Schistosoma haematobium group.Parasitology. 2001;123 Suppl:S245-60. doi: 10.1017/s0031182001008046. Parasitology. 2001. PMID: 11769287 Review.
Cited by
-
Freshwater snail-borne parasitic diseases in Africa.Trop Med Health. 2024 Sep 20;52(1):61. doi: 10.1186/s41182-024-00632-1. Trop Med Health. 2024. PMID: 39304958 Free PMC article. Review.
-
Spatial distribution, abundance, and infection rates of human schistosome-transmitting snails and related physicochemical parameters in KwaZulu-Natal (KZN) province, South Africa.Heliyon. 2022 Dec 21;9(2):e12463. doi: 10.1016/j.heliyon.2022.e12463. eCollection 2023 Feb. Heliyon. 2022. PMID: 36793949 Free PMC article.
-
Identification of Bulinus forskalii as a potential intermediate host of Schistosoma hæmatobium in Senegal.PLoS Negl Trop Dis. 2023 May 9;17(5):e0010584. doi: 10.1371/journal.pntd.0010584. eCollection 2023 May. PLoS Negl Trop Dis. 2023. PMID: 37159452 Free PMC article.
-
Assessment of schistosomiasis transmission in the River Nile at Greater Cairo using malacological surveys and cercariometry.J Parasit Dis. 2022 Dec;46(4):1090-1102. doi: 10.1007/s12639-022-01529-8. Epub 2022 Sep 10. J Parasit Dis. 2022. PMID: 36457778 Free PMC article.
-
Spatial and seasonal distribution of human schistosomiasis intermediate host snails and their interactions with other freshwater snails in 7 districts of KwaZulu-Natal province, South Africa.Sci Rep. 2023 May 15;13(1):7845. doi: 10.1038/s41598-023-34122-x. Sci Rep. 2023. PMID: 37188748 Free PMC article.
References
-
- Goll PH, Wilkins HA. Field studies on Bulinus senegalensis Muller and the transmission of Schistosoma haematobium infection in a Gambian community. Tropenmed Parasitol. 1984; 35(1):29–36. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

