Rapid diagnosis of COVID-19 in the first year of the pandemic: A systematic review
- PMID: 34607235
- PMCID: PMC8440261
- DOI: 10.1016/j.intimp.2021.108144
Rapid diagnosis of COVID-19 in the first year of the pandemic: A systematic review
Abstract
Background: COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a global health threat and remains a challenge for modern medicine. Rapid and accurate diagnosis of COVID-19 is vital for proper disease and outbreak management. Our review aimed to analyze scientific articles published in the literature addressing the rapid tests available for COVID-19 diagnosis at the first year of the pandemic.
Methods: A systematic review was performed from October 22 to 27, 2020, searching data published in PubMed and Google Scholar databases, using subject headings or keywords related to point of care and rapid test diagnostic for SARS-CoV-2 and COVID-19.
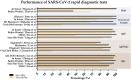
Results: The first survey identified 403 articles, but only 23 met the defined criteria for the systematic analysis. The sensitivity and specificity parameters were assessed in 19 studies, and the data suggested that there was lower sensitivity in the period 1 to 7 days after the emergence of symptoms (∼38%) higher sensitivity at 8 to 14 days (∼90%), and the highest at 15 to 39 days (∼98%). Accuracy was reported in six studies, reporting values above 50%. Only three studies reported a possible cross-reaction.
Conclusions: Our findings indicate that the rapid tests used in the first year of the pandemic were tested with a small number of samples and not adequately validated. And the studies that described them were conducted with little scientific rigor.
Keywords: COVID-19; Point-of-care; Quick test; Rapid Diagnostic test; SARS-CoV-2; Serological test.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- World Health Organization. Pneumonia of unknown cause – China (2020). https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-ch... [Acessed April 20, 2020].
-
- Kang S., Peng W., Zhu Y., Lu S., Zhou M., Lin W., et al. Recent progress in understanding 2019 novel coronavirus (SARS-CoV-2) associated with human respiratory disease: detection, mechanisms and treatment. International Journal of Antimicrobial Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105950. In press. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous