Neutralizing antibody responses following natural SARS-CoV-2 infection: Dynamics and correlation with commercial serologic tests
- PMID: 34607239
- PMCID: PMC8479371
- DOI: 10.1016/j.jcv.2021.104988
Neutralizing antibody responses following natural SARS-CoV-2 infection: Dynamics and correlation with commercial serologic tests
Abstract
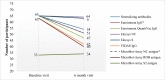
The prediction of SARS-CoV-2 immunity by commercially available serologic tests will be crucial to assess the efficacy of vaccination. We used plaque reduction neutralization testing as the reference standard to evaluate the diagnostic performance of six commercial serologic tests for monitoring SARS-CoV-2 neutralizing antibodies. Euroimmun ELISA anti-spike 1 IgG, Euroimmun anti-spike 1 IgG QuantiVac ELISA, Elecsys Anti-nucleocapsid protein total antibodies, Elecsys Anti-receptor-binding domain total antibodies, VIDAS anti-spike subdomain IgG, and Microblot-Array COVID-19 IgG assay were performed on 228 sera from 89 healthcare workers who participated in a six-month seroprevalence survey. Although all immunoassays demonstrated similar performances, VIDAS SARS-CoV-2 IgG and Euroimmun QuantiVac IgG (area under the curve 0.96 and 0.95 respectively) showed the better ability to detect Nabs. Except for the Elecsys Anti-SARS-CoV-2 and the Elecsys Anti-SARS-CoV-2 S assays, the commercial serologic tests evaluated here showed a significant decrease of antibody titers in the 6-month follow-up samples. Depending on the immunoassay, 21% to 33% of the participants became seronegative, and 16.9% had a loss of neutralizing antibodies. Microblot-Array assay results showed cross-reactivity with HCoVNL63 in only one sample, and this sample showed SARS-CoV-2 neutralizing capacity. In conclusion, our results support the use of VIDAS SARS-CoV-2 IgG, Euroimmun Anti-SARS-CoV-2 ELISA IgG, Euroimmun Anti-SARS-CoV-2 QuantiVac ELISA IgG and Microblot-Array COVID-19 IgG assays to monitor neutralizing antibody response following natural SARS-CoV-2 infection. These immunoassays could facilitate the prediction of post-vaccine protection in the long term and the allocation of booster doses.
Keywords: Immunoassays; Neutralizing antibodies; SARS-CoV-2.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
bioMérieux has partly sponsored this study. EuroImmun (Euroimmun Anti-SARS-CoV-2 IgG QuantiVac ELISA) and TestLine CliniCal Diagnostics (Microblot-array COVID-19 IgG assay) have offered reagents for validation. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Figures
References
-
- Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M., Cordes S., Ross B., Esser S., Lindemann M., Kribben A., Dittmer U., Witzke O., Herrmann A. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. 2020;128 - PMC - PubMed
-
- Pollán M., Pérez-Gómez B., Pastor-Barriuso R., Oteo J., Hernán M.A., Pérez-Olmeda M., Sanmartín J.L., Fernández-García A., Cruz I., Fernández de Larrea N., Molina M., Rodríguez-Cabrera F., Martín M., Merino-Amador P., León Paniagua J., Muñoz-Montalvo J.F., Blanco F., Yotti R., ENE-COVID Study Group Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. - PMC - PubMed
-
- Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.D., Hall A.J., Fry A.M., Cannon D.L., Chiang C.F., Gibbons A., Krapiunaya I., Morales-Betoulle M., Roguski K., Rasheed M.A.U., Freeman B., Lester S., Mills L., Carroll D.S., Owen S.M., Johnson J.A., Semenova V., Blackmore C., Blog D., Chai S.J., Dunn A., Hand J., Jain S., Lindquist S., Lynfield R., Pritchard S., Sokol T., Sosa L., Turabelidze G., Watkins S.M., Wiesman J., Williams R.W., Yendell S., Schiffer J., Thornburg N.J. 21 July 2020. Seroprevalence of Antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020 Jul 21. doi: 10.1001/jamainternmed.2020.4130. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous