Effects of periostin deficiency on kidney aging and lipid metabolism
- PMID: 34607314
- PMCID: PMC8544301
- DOI: 10.18632/aging.203580
Effects of periostin deficiency on kidney aging and lipid metabolism
Abstract
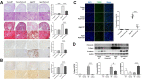
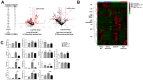
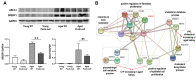
Periostin plays a crucial role in fibrosis, which is involved in kidney aging. A few studies have shown that lipid metabolism is involved in kidney aging. We investigated the role of periostin in lipid metabolism during kidney aging. Renal function, fibrosis, and inflammatory markers were studied using urine, blood, and tissue samples from wild-type (WT) C57BL/6 mice and Postn-null mice of 2 and 24 months of age. Lipids were quantitatively profiled using liquid chromatography-tandem mass spectrometry in the multiple reaction monitoring mode. Renal function was worse and tubular atrophy/interstitial fibrosis, periostin expression, and inflammatory and fibrotic markers were more severe in aged WT mice than in young WT mice. In aged Postn-null mice, these changes were mitigated. Thirty-five differentially regulated lipids were identified. Phosphatidylcholines, cholesteryl ester, cholesterol, ceramide-1-phosphate, and CCL5 expression were significantly higher in aged WT mice than in aged Postn-null mice. Particularly, linoleic acid, linolenic acid, arachidonic acid, and docosahexaenoic acid differed strongly between the two groups. Lysophosphatidylcholine acyltransferase 2, which converts lysophosphatidylcholine to phosphatidylcholine, was significantly higher in aged WT mice than in aged Postn-null mice. Periostin expression in the kidneys increased with age, and periostin ablation delayed aging. Changes in lipids and their metabolism were found in Postn-null mice. Further research on the precise mechanisms of and relationships between lipid expression and metabolism, kidney aging, and periostin expression is warranted.
Keywords: aging; kidney; lipid; lipidomics; periostin.
Conflict of interest statement
Figures


Similar articles
-
Experimental Inhibition of Periostin Attenuates Kidney Fibrosis.Am J Nephrol. 2017;46(6):501-517. doi: 10.1159/000485325. Epub 2017 Dec 21. Am J Nephrol. 2017. PMID: 29268247
-
Blocking Periostin Prevented Development of Inflammation in Rhabdomyolysis-Induced Acute Kidney Injury Mice Model.Cells. 2022 Oct 27;11(21):3388. doi: 10.3390/cells11213388. Cells. 2022. PMID: 36359784 Free PMC article.
-
Periostin induces kidney fibrosis after acute kidney injury via the p38 MAPK pathway.Am J Physiol Renal Physiol. 2019 Mar 1;316(3):F426-F437. doi: 10.1152/ajprenal.00203.2018. Epub 2018 Dec 12. Am J Physiol Renal Physiol. 2019. PMID: 30539653
-
Periostin in skin tissue and skin-related diseases.Allergol Int. 2014 Jun;63(2):161-70. doi: 10.2332/allergolint.13-RAI-0685. Epub 2014 Apr 25. Allergol Int. 2014. PMID: 24759560 Review.
-
Periostin in the Kidney.Adv Exp Med Biol. 2019;1132:99-112. doi: 10.1007/978-981-13-6657-4_11. Adv Exp Med Biol. 2019. PMID: 31037629 Review.
Cited by
-
Periostin deficiency attenuates kidney fibrosis in diabetic nephropathy by improving pancreatic β-cell dysfunction and reducing kidney EMT.Sci Rep. 2023 Oct 16;13(1):17599. doi: 10.1038/s41598-023-44177-5. Sci Rep. 2023. PMID: 37845302 Free PMC article.
-
Constitutive activation mechanism of a class C GPCR.Nat Struct Mol Biol. 2024 Apr;31(4):678-687. doi: 10.1038/s41594-024-01224-7. Epub 2024 Feb 8. Nat Struct Mol Biol. 2024. PMID: 38332368
-
Deficiency for scavenger receptors Stabilin-1 and Stabilin-2 leads to age-dependent renal and hepatic depositions of fasciclin domain proteins TGFBI and Periostin in mice.Aging Cell. 2023 Sep;22(9):e13914. doi: 10.1111/acel.13914. Epub 2023 Jun 25. Aging Cell. 2023. PMID: 37357460 Free PMC article.
-
Arachidonic acid in aging: New roles for old players.J Adv Res. 2025 Apr;70:79-101. doi: 10.1016/j.jare.2024.05.003. Epub 2024 May 4. J Adv Res. 2025. PMID: 38710468 Free PMC article. Review.
References
-
- Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, Shikotra A, Carter R, Audusseau S, Hamid Q, Bradding P, Fahy JV, Woodruff PG, et al., and Bronchoscopic Exploratory Research Study of Biomarkers in Corticosteroid-refractory Asthma (BOBCAT) Study Group. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012; 130:647–54.e10. 10.1016/j.jaci.2012.06.025 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

