Long term follow-up of a phase II study of cladribine with concurrent rituximab with hairy cell leukemia variant
- PMID: 34607348
- PMCID: PMC9153043
- DOI: 10.1182/bloodadvances.2021005039
Long term follow-up of a phase II study of cladribine with concurrent rituximab with hairy cell leukemia variant
Abstract
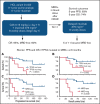
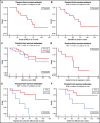
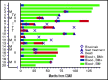
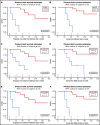
Hairy cell leukemia variant (HCLv) responds poorly to purine analogue monotherapy. Rituximab concurrent with cladribine (CDAR) improves response rates, but long-term outcomes are unknown. We report final results of a phase 2 study of CDAR for patients with HCLv. Twenty patients with 0 to 1 prior courses of cladribine and/or rituximab, including 8 who were previously untreated, received cladribine 0.15 mg/kg on days 1 to 5 with 8 weekly rituximab doses of 375 mg/m2 beginning day 1. Patients received a second rituximab course ≥6 months after cladribine, if and when minimal residual disease (MRD) was detected in blood. The complete remission (CR) rate from CDAR was 95% (95% confidence interval, 75-100). Sixteen (80%) of 20 patients (95% confidence interval, 56-94) became MRD negative according to bone marrow at 6 months. The median duration of MRD-negative CR was 70.1 months, and 7 of 16 are still MRD negative up to 120 months. With a median follow-up of 69.7 months, 11 patients received delayed rituximab, and the 5-year progression-free survival (PFS) and overall survival (OS) were 63.3% and 73.9%, respectively. Five patients with TP53 mutations had shorter PFS (median, 36.4 months vs unreached; P = .0024) and OS (median, 52.4 months vs unreached; P = .032). MRD-negative CR at 6 months was significantly associated with longer PFS (unreached vs 17.4 months; P < .0001) and OS (unreached vs 38.2 months; P < .0001). Lack of MRD in blood at 6 months was also predictive of longer PFS and OS (P < .0001). After progression following CDAR, median OS was 29.7 months. CDAR is effective in HCLv, with better outcomes in patients who achieve MRD-negative CR. This trial is registered at www.clinicaltrials.gov as #NCT00923013.
© 2021 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures
References
-
- Matutes E, Martínez-Trillos A, Campo E.. Hairy cell leukaemia-variant: disease features and treatment. Best Pract Res Clin Haematol. 2015;28(4):253-263. - PubMed
-
- Matutes E, Wotherspoon A, Brito-Babapulle V, Catovsky D.. The natural history and clinico-pathological features of the variant form of hairy cell leukemia. Leukemia. 2001;15(1):184-186. - PubMed
-
- Robak T. Hairy-cell leukemia variant: recent view on diagnosis, biology and treatment. Cancer Treat Rev. 2011;37(1):3-10. - PubMed
-
- Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR.. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

