A Myosin Light Chain Is Critical for Fungal Growth Robustness in Candida albicans
- PMID: 34607458
- PMCID: PMC8546852
- DOI: 10.1128/mBio.02528-21
A Myosin Light Chain Is Critical for Fungal Growth Robustness in Candida albicans
Abstract
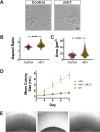
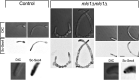
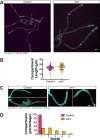
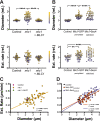
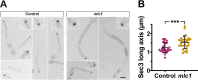
In a number of elongated cells, such as fungal hyphae, a vesicle cluster is observed at the growing tip. This cluster, called a Spitzenkörper, has been suggested to act as a vesicle supply center, yet analysis of its function is challenging, as a majority of components identified thus far are essential for growth. Here, we probe the function of the Spitzenkörper in the human fungal pathogen Candida albicans, using genetics and synthetic physical interactions (SPI). We show that the C. albicans Spitzenkörper is comprised principally of secretory vesicles. Mutant strains lacking the Spitzenkörper component myosin light chain 1 (Mlc1) or having a SPI between Mlc1 and either another Spitzenkörper component, the Rab GTPase Sec4, or prenylated green fluorescent protein (GFP), are viable and still exhibit a Spitzenkörper during filamentous growth. Strikingly, all of these mutants formed filaments with increased diameters and extension rates, indicating that Mlc1 negatively regulates myosin V, Myo2, activity. The results of our quantitative studies reveal a strong correlation between filament diameter and extension rate, which is consistent with the vesicle supply center model for fungal tip growth. Together, our results indicate that the Spitzenkörper protein Mlc1 is important for growth robustness and reveal a critical link between filament morphology and extension rate. IMPORTANCE Hyphal tip growth is critical in a range of fungal pathogens, in particular for invasion into animal and plant tissues. In Candida albicans, as in many filamentous fungi, a cluster of vesicles, called a Spitzenkörper, is observed at the tip of growing hyphae that is thought to function as a vesicle supply center. A central prediction of the vesicle supply center model is that the filament diameter is proportional to the extension rate. Here, we show that mutants lacking the Spitzenkörper component myosin light chain 1 (Mlc1) or having synthetic physical interactions between Mlc1 and either another Spitzenkörper component or prenylated GFP, are defective in filamentous growth regulation, exhibiting a range of growth rates and sizes, with a strong correlation between diameter and extension rate. These results suggest that the Spitzenkörper is important for growth robustness and reveal a critical link between filament morphology and extension rate.
Keywords: Spitzenkörper; filamentous growth; morphology; secretion.
Figures





References
-
- Girbardt M. 1957. Der Spitzenkörper von Polystictus versicolor (L.). Planta 50:47–59. doi:10.1007/BF01912343. - DOI
-
- Riquelme M, Aguirre J, Bartnicki-García S, Braus GH, Feldbrügge M, Fleig U, Hansberg W, Herrera-Estrella A, Kämper J, Kück U, Mouriño-Pérez RR, Takeshita N, Fischer R. 2018. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol Mol Biol Rev 82:e00068-17. doi:10.1128/MMBR.00068-17. - DOI - PMC - PubMed
-
- Bartnicki-Garcia S, Hergert F, Gierz G. 1989. Computer simulation of fungal morphogenesis and the mathematical basis for hyphal (tip) growth. Protoplasma 153:46–57. doi:10.1007/BF01322464. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

