Revisiting the Role of Toxoplasma gondii ERK7 in the Maintenance and Stability of the Apical Complex
- PMID: 34607461
- PMCID: PMC8546650
- DOI: 10.1128/mBio.02057-21
Revisiting the Role of Toxoplasma gondii ERK7 in the Maintenance and Stability of the Apical Complex
Abstract
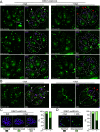
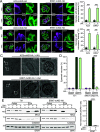
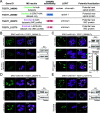
Toxoplasma gondii extracellular signal-regulated kinase 7 (ERK7) is known to contribute to the integrity of the apical complex and to participate in the final step of conoid biogenesis. In the absence of ERK7, mature parasites lose their conoid complex and are unable to glide, invade, or egress from host cells. In contrast to a previous report, we show here that the depletion of ERK7 phenocopies the depletion of the apical cap protein AC9 or AC10. The absence of ERK7 leads to the loss of the apical polar ring (APR), the disorganization of the basket of subpellicular microtubules (SPMTs), and a severe impairment in microneme secretion. Ultrastructure expansion microscopy (U-ExM), coupled to N-hydroxysuccinimide ester (NHS-ester) staining on intracellular parasites, offers an unprecedented level of resolution and highlights the disorganization of the rhoptries as well as the dilated plasma membrane at the apical pole in the absence of ERK7. Comparative proteomics analysis of wild-type and ERK7-depleted parasites confirmed the disappearance of known apical complex proteins, including markers of the apical polar ring and a new apical cap named AC11. Concomitantly, the absence of ERK7 led to an accumulation of microneme proteins, resulting from the defect in the exocytosis of the organelles. AC9-depleted parasites were included as controls and exhibited an increase in inner membrane complex proteins, with two new proteins assigned to this compartment, namely, IMC33 and IMC34. IMPORTANCE The conoid is an enigmatic, dynamic organelle positioned at the apical tip of the coccidian subgroup of the Apicomplexa, close to the apical polar ring (APR) from which the subpellicular microtubules (SPMTs) emerge and through which the secretory organelles (micronemes and rhoptries) reach the plasma membrane for exocytosis. In Toxoplasma gondii, the conoid protrudes concomitantly with microneme secretion, during egress, motility, and invasion. The conditional depletion of the apical cap structural protein AC9 or AC10 leads to a disorganization of SPMTs as well as the loss of the APR and conoid, resulting in a microneme secretion defect and a block in motility, invasion, and egress. We show here that the depletion of the kinase ERK7 phenocopies AC9 and AC10 mutants. The combination of ultrastructure expansion microscopy and NHS-ester staining revealed that ERK7-depleted parasites exhibit a dilated apical plasma membrane and an altered positioning of the rhoptries, while electron microscopy images unambiguously highlight the loss of the APR.
Keywords: Apicomplexa; Toxoplasma gondii; apical cap; apicomplexan parasites; comparative proteomics; conoid; egress; extracellular signal-regulated kinase; host cell invasion; invasion; microneme secretion; microtubule; motility; subpellicular microtubules.
Figures




Similar articles
-
Essential function of the alveolin network in the subpellicular microtubules and conoid assembly in Toxoplasma gondii.Elife. 2020 May 7;9:e56635. doi: 10.7554/eLife.56635. Elife. 2020. PMID: 32379047 Free PMC article.
-
Ancient MAPK ERK7 is regulated by an unusual inhibitory scaffold required for Toxoplasma apical complex biogenesis.Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):12164-12173. doi: 10.1073/pnas.1921245117. Epub 2020 May 14. Proc Natl Acad Sci U S A. 2020. PMID: 32409604 Free PMC article.
-
Multivalent Interactions Drive the Toxoplasma AC9:AC10:ERK7 Complex To Concentrate ERK7 in the Apical Cap.mBio. 2021 Feb 22;13(1):e0286421. doi: 10.1128/mbio.02864-21. Epub 2022 Feb 8. mBio. 2021. PMID: 35130732 Free PMC article.
-
Nanoscale imaging of the conoid and functional dissection of its dynamics in Apicomplexa.Curr Opin Microbiol. 2022 Dec;70:102226. doi: 10.1016/j.mib.2022.102226. Epub 2022 Nov 2. Curr Opin Microbiol. 2022. PMID: 36332501 Review.
-
Evolution, Composition, Assembly, and Function of the Conoid in Apicomplexa.Trends Parasitol. 2020 Aug;36(8):688-704. doi: 10.1016/j.pt.2020.05.001. Epub 2020 May 31. Trends Parasitol. 2020. PMID: 32487504 Review.
Cited by
-
Bumped Kinase Inhibitors Inhibit both Toxoplasma gondii MAPKL1 and CDPK1.ACS Infect Dis. 2025 Jun 13;11(6):1552-1562. doi: 10.1021/acsinfecdis.5c00051. Epub 2025 May 23. ACS Infect Dis. 2025. PMID: 40407244
-
Conoid extrusion regulates glideosome assembly to control motility and invasion in Apicomplexa.Nat Microbiol. 2022 Nov;7(11):1777-1790. doi: 10.1038/s41564-022-01212-x. Epub 2022 Sep 15. Nat Microbiol. 2022. PMID: 36109645
-
SPARK regulates AGC kinases central to the Toxoplasma gondii asexual cycle.bioRxiv [Preprint]. 2024 May 2:2023.10.30.564746. doi: 10.1101/2023.10.30.564746. bioRxiv. 2024. Update in: Elife. 2024 Aug 13;13:RP93877. doi: 10.7554/eLife.93877. PMID: 37961644 Free PMC article. Updated. Preprint.
-
Structural and Functional Insights into the Microtubule Organizing Centers of Toxoplasma gondii and Plasmodium spp.Microorganisms. 2021 Dec 3;9(12):2503. doi: 10.3390/microorganisms9122503. Microorganisms. 2021. PMID: 34946106 Free PMC article. Review.
-
The initiation and early development of the tubulin-containing cytoskeleton in the human parasite Toxoplasma gondii.Mol Biol Cell. 2024 Mar 1;35(3):ar37. doi: 10.1091/mbc.E23-11-0418. Epub 2024 Jan 3. Mol Biol Cell. 2024. PMID: 38170577 Free PMC article.
References
-
- Back PS, O’Shaughnessy WJ, Moon AS, Dewangan PS, Hu X, Sha J, Wohlschlegel JA, Bradley PJ, Reese ML. 2020. Ancient MAPK ERK7 is regulated by an unusual inhibitory scaffold required for Toxoplasma apical complex biogenesis. Proc Natl Acad Sci USA 117:12164–12173. doi:10.1073/pnas.1921245117. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

