Added Value of a Blinded Outcome Adjudication Committee in an Open-Label Randomized Stroke Trial
- PMID: 34607469
- PMCID: PMC8700318
- DOI: 10.1161/STROKEAHA.121.035301
Added Value of a Blinded Outcome Adjudication Committee in an Open-Label Randomized Stroke Trial
Abstract
Background and purpose: Blinded outcome assessment in trials with prospective randomized open blinded end point design is challenging. Unblinding can result in misclassified outcomes and biased treatment effect estimates. An outcome adjudication committee assures blinded outcome assessment, but the added value for trials with prospective randomized open blinded end point design and subjective outcomes is unknown. We aimed to assess the degree of misclassification of modified Rankin Scale (mRS) scores by a central assessor and its impact on treatment effect estimates in a stroke trial with prospective randomized open blinded end point design.
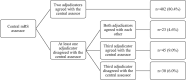
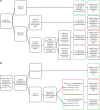
Methods: We used data from the MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands). The primary outcome was the mRS at 90 days. Standardized, algorithm-based telephone interviews to assess the mRS were conducted from a central location by an experienced research nurse, unaware but not formally blinded to treatment allocation (central assessor). Masked reports of these interviews were adjudicated by a blinded outcome committee. Misclassification was defined as an incorrect classification of the mRS by the central assessor. The effect of endovascular treatment on the mRS was assessed with multivariable ordinal logistic regression.
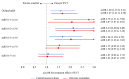
Results: In MR CLEAN, 53/500 (10.6%) of the mRS scores were misclassified. The degree and direction of misclassification did not differ between treatment arms (P=0.59). Benefit of endovascular treatment was shown on the mRS when scored by the central assessor (adjusted common odds ratio, 1.60 [95% CI, 1.16-2.21]) and the outcome adjudication committee (adjusted common odds ratio, 1.67 [95% CI, 1.21-2.20]).
Conclusions: Misclassification by the central assessor was small, randomly distributed over treatment arms, and did not affect treatment effect estimates. This study suggests that the added value of a blinded outcome adjudication committee is limited in a stroke trial with prospective randomized open blinded end point design applying standardized, algorithm-based outcome assessment by a central assessor, who is unaware but not formally blinded to treatment allocation. Registration: URL: https://www.isrctn.com; Unique identifier: ISRCTN10888758.
Keywords: algorithm; clinical trial; ischemic stroke; odds ratio; telephone.
Figures

References
-
- Quinn TJ, Dawson J, Walters MR, Lees KR. Functional outcome measures in contemporary stroke trials. Int J Stroke. 2009;4:200–205. doi: 10.1111/j.1747-4949.2009.00271.x - PubMed
-
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604 - PubMed
-
- Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin Scale: a systematic review. Stroke. 2009;40:3393–3395. doi: 10.1161/STROKEAHA.109.557256 - PubMed
-
- Hansson L, Hedner T, Dahlöf B. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Prospective randomized open blinded end-point. Blood Press. 1992;1:113–119. doi: 10.3109/08037059209077502 - PubMed
-
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, Ravaud P, Brorson S. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. BMJ. 2012;344:e1119. doi: 10.1136/bmj.e1119 - PubMed

