Recent findings and applications of biomedical engineering for COVID-19 diagnosis: a critical review
- PMID: 34607509
- PMCID: PMC8806999
- DOI: 10.1080/21655979.2021.1987821
Recent findings and applications of biomedical engineering for COVID-19 diagnosis: a critical review
Abstract
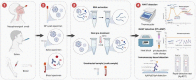
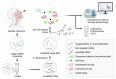
COVID-19 is one of the most severe global health crises that humanity has ever faced. Researchers have restlessly focused on developing solutions for monitoring and tracing the viral culprit, SARS-CoV-2, as vital steps to break the chain of infection. Even though biomedical engineering (BME) is considered a rising field of medical sciences, it has demonstrated its pivotal role in nurturing the maturation of COVID-19 diagnostic technologies. Within a very short period of time, BME research applied to COVID-19 diagnosis has advanced with ever-increasing knowledge and inventions, especially in adapting available virus detection technologies into clinical practice and exploiting the power of interdisciplinary research to design novel diagnostic tools or improve the detection efficiency. To assist the development of BME in COVID-19 diagnosis, this review highlights the most recent diagnostic approaches and evaluates the potential of each research direction in the context of the pandemic.
Keywords: COVID-19; CRISPR; RT-PCR; biomedical engineering; diagnosis; iNAAT; immunoassay; microfluidic devices.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
A review on current diagnostic techniques for COVID-19.Expert Rev Mol Diagn. 2021 Feb;21(2):141-160. doi: 10.1080/14737159.2021.1886927. Epub 2021 Mar 18. Expert Rev Mol Diagn. 2021. PMID: 33593219 Review.
-
Rapid diagnosis of SARS-CoV-2 using potential point-of-care electrochemical immunosensor: Toward the future prospects.Int Rev Immunol. 2021;40(1-2):126-142. doi: 10.1080/08830185.2021.1872566. Epub 2021 Jan 15. Int Rev Immunol. 2021. PMID: 33448909 Review.
-
Novel approaches for rapid detection of COVID-19 during the pandemic: A review.Anal Biochem. 2021 Dec 1;634:114362. doi: 10.1016/j.ab.2021.114362. Epub 2021 Aug 31. Anal Biochem. 2021. PMID: 34478703 Free PMC article. Review.
-
Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection.Clin Microbiol Rev. 2021 May 12;34(3):e00228-20. doi: 10.1128/CMR.00228-20. Print 2021 Jun 16. Clin Microbiol Rev. 2021. PMID: 33980687 Free PMC article. Review.
-
Recent advances in methods for the diagnosis of Corona Virus Disease 2019.J Clin Lab Anal. 2022 Jan;36(1):e24178. doi: 10.1002/jcla.24178. Epub 2021 Dec 17. J Clin Lab Anal. 2022. PMID: 34921443 Free PMC article. Review.
Cited by
-
Molecular docking and molecular dynamics study Lianhua Qingwen granules (LHQW) treats COVID-19 by inhibiting inflammatory response and regulating cell survival.Front Cell Infect Microbiol. 2022 Nov 24;12:1044770. doi: 10.3389/fcimb.2022.1044770. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36506032 Free PMC article.
-
Update on the Development of Toehold Switch-Based Approach for Molecular Diagnostic Tests of COVID-19.J Nucleic Acids. 2022 May 9;2022:7130061. doi: 10.1155/2022/7130061. eCollection 2022. J Nucleic Acids. 2022. PMID: 35586794 Free PMC article. Review.
-
Diagnostic value of BinaxNOW antigen card for Severe Acute Respiratory Syndrome Coronavirus 2.Bioengineered. 2023 Dec;14(1):2180221. doi: 10.1080/21655979.2023.2180221. Bioengineered. 2023. PMID: 37489712 Free PMC article.
-
Planning and Budgeting of Medical Devices Among Ethiopian Public Hospitals.Clinicoecon Outcomes Res. 2022 May 19;14:405-413. doi: 10.2147/CEOR.S363376. eCollection 2022. Clinicoecon Outcomes Res. 2022. PMID: 35615660 Free PMC article.
-
Effects of infection prevention and control measures on patient and visitor violence against health workers in China during COVID-19 pandemic.Front Public Health. 2023 Jun 5;11:1140561. doi: 10.3389/fpubh.2023.1140561. eCollection 2023. Front Public Health. 2023. PMID: 37342269 Free PMC article.
References
-
- Worldometers . COVID-19 Coronavirus Pandemic. 2021.
-
- Rebecca MN, Sutter KM, Sutherland MD.. Global economic effects of COVID-19. Congressional Research Service; 2021. https://fas.org/sgp/crs/row/R46270.pdf
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
