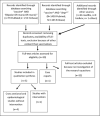
Outcomes associated with different vaccines in individuals with bipolar disorder and impact on the current COVID-19 pandemic- a systematic review
- PMID: 34607722
- PMCID: PMC8429356
- DOI: 10.1016/j.euroneuro.2021.09.001
Outcomes associated with different vaccines in individuals with bipolar disorder and impact on the current COVID-19 pandemic- a systematic review
Abstract
Bipolar disorder (BD) might be associated with higher infection rates of coronavirus disease (COVID-19) which in turn could result in worsening the clinical course and outcome. This may be due to a high prevalence of somatic comorbidities and an increased risk of delays in and poorer treatment of somatic disease in patients with severe mental illness in general. Vaccination is the most important public health intervention to tackle the ongoing pandemic. We undertook a systematic review regarding the data on vaccinations in individuals with BD. Proportion of prevalence rates, efficacy and specific side effects of vaccinations and in individuals with BD were searched. Results show that only five studies have investigated vaccinations in individuals with BD, which substantially limits the interpretation of overall findings. Studies on antibody production after vaccinations in BD are very limited and results are inconsistent. Also, the evidence-based science on side effects of vaccinations in individuals with BD so far is poor.
Keywords: Bipolar disorder; COVID-19; Infectious diseases; Systematic review; Vaccination.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest As the article is a review on the literature, there is generally no conflict of interest in respect to this article. Nevertheless, some of the authors had relationships with different companies or served as consultants in the last three years. MM has served as consultant, or CME speaker for Angelini. EV has received grants and served as consultant, advisor or CME speaker for the following entities: AB-Biotics, Abbott, AbbVie, Angelini, Boehringer-Ingelheim, Dainippon Sumitomo Pharma, Ferrer, Gedeon Richter, GH Research, Janssen, Lundbeck, Novartis, Otsuka, Sage, Sanofi-Aventis, Sunovion, and Takeda, outside the submitted work. LM declares that he has received lecture honoraria from Lundbeck pharmaceutical. No other equity ownership, profit-sharing agreements, royalties, or patent. CC has been a consultant and/or advisor to or has received honoraria from: Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Rovi, Supernus, and Teva. He has received grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma. AF is /has been a consultant and/or a speaker and/or has received research grants from Angelini, Apsen, Boheringer Ingelheim, Daiichi Sankyo, Doc Generici, Glaxo Smith Kline, Italfarmaco, Lundbeck, Janssen, Mylan, Neuraxpharm, Otsuka, Pfizer, Recordati, Sanofi Aventis, Sunovion, Vifor. MB has received institutional grants from Deutsche Forschungsgemeinschaft (DFG), Bundesministerium für Bildung und Forschung (BMBF), and European Commission. He served in advisory boards for GH Research, Janssen-Cilag, neuraxpharm, Novartis, Sandoz, Shire International, Sumitomo Dainippon, Sunovion, and Takeda, and received speaker honoraria for Aristo, Hexal, Janssen Pharmaceutica NV, Janssen-Cilag, and Sunovion, outside the submitted work. REN has received research grants from H. Lundbeck and Otsuka Pharmaceuticals for clinical trials, received speaking fees from Bristol-Myers Squibb, Astra Zeneca, Janssen & Cilag, Lundbeck, Servier, Otsuka Pharmaceuticals, Teva A/S, and Eli Lilly and has acted as advisor to Astra Zeneca, Eli Lilly, Lundbeck, Otsuka Pharmaceuticals, Takeda, and Medivir, and has acted as investigator for Janssen-Cilag, Lundbeck, Boehringer, Compass and Sage. RW Licht has within the preceding three years served an advisory board of Janssen Cilag and Sage and has received speaker honorarium from Astra-Zeneca, Jannsen-Cilag, Servier, Lundbeck and Teva. No known conflicts of interest in respect to this article from all other authors.
Figures
Similar articles
-
Implications of the COVID-19 pandemic for people with bipolar disorders: A scoping review.J Affect Disord. 2021 Dec 1;295:740-751. doi: 10.1016/j.jad.2021.08.091. Epub 2021 Sep 4. J Affect Disord. 2021. PMID: 34517248 Free PMC article.
-
Psychological and behavioral response on the COVID-19 pandemic in individuals with bipolar disorder: A multicenter study.Psychiatry Res. 2022 Apr;310:114451. doi: 10.1016/j.psychres.2022.114451. Epub 2022 Feb 16. Psychiatry Res. 2022. PMID: 35190338 Free PMC article.
-
Effects of the COVID-19 pandemic in a preexisting longitudinal study of patients with recently diagnosed bipolar disorder: Indications for increases in manic symptoms.Brain Behav. 2021 Nov;11(11):e2326. doi: 10.1002/brb3.2326. Epub 2021 Sep 23. Brain Behav. 2021. PMID: 34554650 Free PMC article.
-
COVID-19-related fears and information frequency predict sleep behavior in bipolar disorder.Brain Behav. 2021 Sep;11(9):e02182. doi: 10.1002/brb3.2182. Epub 2021 Aug 19. Brain Behav. 2021. PMID: 34409763 Free PMC article.
-
Prevalence of posttraumatic stress disorder after infectious disease pandemics in the twenty-first century, including COVID-19: a meta-analysis and systematic review.Mol Psychiatry. 2021 Sep;26(9):4982-4998. doi: 10.1038/s41380-021-01036-x. Epub 2021 Feb 4. Mol Psychiatry. 2021. PMID: 33542468 Free PMC article.
Cited by
-
The Network of Services for COVID-19 Vaccination in Persons With Mental Disorders: The Italian Social Health System, Its Organization, and Bioethical Issues.Front Public Health. 2022 Jun 20;10:870386. doi: 10.3389/fpubh.2022.870386. eCollection 2022. Front Public Health. 2022. PMID: 35795707 Free PMC article. Review.
-
Greater Emotional Distress Due to Social Distancing and Greater Symptom Severity during the COVID-19 Pandemic in Individuals with Bipolar Disorder: A Multicenter Study in Austria, Germany, and Denmark.Int J Environ Res Public Health. 2022 Jun 22;19(13):7626. doi: 10.3390/ijerph19137626. Int J Environ Res Public Health. 2022. PMID: 35805284 Free PMC article.
-
Psychotropic drug repurposing for COVID-19: A Systematic Review and Meta-Analysis.Eur Neuropsychopharmacol. 2023 Jan;66:30-44. doi: 10.1016/j.euroneuro.2022.10.004. Epub 2022 Oct 20. Eur Neuropsychopharmacol. 2023. PMID: 36399837 Free PMC article.
References
-
- Anderson G., Berk M., Dean O., Moylan S., Maes M. Role of immune-inflammatory and oxidative and nitrosative stress pathways in the etiology of depression: therapeutic implications. CNS Drugs. 2014;28(1):1–10. - PubMed
-
- Arbelo N., López-Pelayo H., Sagué M., Madero S., Pinzón-Espinosa J., Gomes-da-Costa S., Ilzarbe L., Anmella G., Llach C.D., Imaz M.L., Cámara M.M., Pintor L. Psychiatric Clinical Profiles and Pharmacological Interactions in COVID-19 Inpatients Referred to a Consultation Liaison Psychiatry Unit: a Cross-Sectional Study. Psychiatr. Q. 2021:1–13. - PMC - PubMed
-
- Barber S., Reed L., Syam N., Jones N., 2020. Severe mental illness and risks from COVID-19, Available from: https://www.cebm.net/covid-19/severe-mental-illness-and-risks-from-covid....