Predicting cardiac adverse events in patients receiving immune checkpoint inhibitors: a machine learning approach
- PMID: 34607896
- PMCID: PMC8491414
- DOI: 10.1136/jitc-2021-002545
Predicting cardiac adverse events in patients receiving immune checkpoint inhibitors: a machine learning approach
Erratum in
-
Correction: Predicting cardiac adverse events in patients receiving immune checkpoint inhibitors: a machine learning approach.J Immunother Cancer. 2021 Oct;9(10):e002545corr1. doi: 10.1136/jitc-2021-002545corr1. J Immunother Cancer. 2021. PMID: 34645683 Free PMC article. No abstract available.
Abstract
Background: Treatment with immune checkpoint inhibitors (ICIs) has been associated with an increased rate of cardiac events. There are limited data on the risk factors that predict cardiac events in patients treated with ICIs. Therefore, we created a machine learning (ML) model to predict cardiac events in this at-risk population.
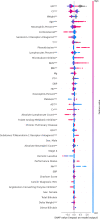
Methods: We leveraged the CancerLinQ database curated by the American Society of Clinical Oncology and applied an XGBoosted decision tree to predict cardiac events in patients taking programmed death receptor-1 (PD-1) or programmed death ligand-1 (PD-L1) therapy. All curated data from patients with non-small cell lung cancer, melanoma, and renal cell carcinoma, and who were prescribed PD-1/PD-L1 therapy between 2013 and 2019, were used for training, feature interpretation, and model performance evaluation. A total of 356 potential risk factors were included in the model, including elements of patient medical history, social history, vital signs, common laboratory tests, oncological history, medication history and PD-1/PD-L1-specific factors like PD-L1 tumor expression.
Results: Our study population consisted of 4960 patients treated with PD-1/PD-L1 therapy, of whom 418 had a cardiac event. The following were key predictors of cardiac events: increased age, corticosteroids, laboratory abnormalities and medications suggestive of a history of heart disease, the extremes of weight, a lower baseline or on-treatment percentage of lymphocytes, and a higher percentage of neutrophils. The final model predicted cardiac events with an area under the curve-receiver operating characteristic of 0.65 (95% CI 0.58 to 0.75). Using our model, we divided patients into low-risk and high-risk subgroups. At 100 days, the cumulative incidence of cardiac events was 3.3% in the low-risk group and 6.1% in the high-risk group (p<0.001).
Conclusions: ML can be used to predict cardiac events in patients taking PD-1/PD-L1 therapy. Cardiac risk was driven by immunological factors (eg, percentage of lymphocytes), oncological factors (eg, low weight), and a cardiac history.
Keywords: immunotherapy; lung neoplasms; programmed cell death 1 receptor.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: TGN has received advisory fees from BMS, H3 Biomedicine, Amgen, AbbVie, and Intrinsic Imaging and grant support from Astra Zeneca.
Figures


Similar articles
-
Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis.JAMA Netw Open. 2019 Jul 3;2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879. JAMA Netw Open. 2019. PMID: 31290993 Free PMC article.
-
Clinical decision support algorithm based on machine learning to assess the clinical response to anti-programmed death-1 therapy in patients with non-small-cell lung cancer.Eur J Cancer. 2021 Aug;153:179-189. doi: 10.1016/j.ejca.2021.05.019. Epub 2021 Jun 26. Eur J Cancer. 2021. PMID: 34182269
-
Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/ Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: A multicentre analysis of immune-related adverse events.Eur J Cancer. 2020 Mar;128:17-26. doi: 10.1016/j.ejca.2019.12.031. Epub 2020 Mar 5. Eur J Cancer. 2020. PMID: 32109847
-
Risk of Ophthalmic Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis.Ocul Immunol Inflamm. 2022 Aug;30(6):1449-1459. doi: 10.1080/09273948.2021.1890133. Epub 2021 May 10. Ocul Immunol Inflamm. 2022. PMID: 33970759
-
The Predictive Value of Programmed Death Ligand 1 in Patients with Metastatic Renal Cell Carcinoma Treated with Immune-checkpoint Inhibitors: A Systematic Review and Meta-analysis.Eur Urol. 2021 Jun;79(6):783-792. doi: 10.1016/j.eururo.2020.10.006. Epub 2020 Nov 7. Eur Urol. 2021. PMID: 33172722
Cited by
-
Cardiac imaging techniques for the assessment of immune checkpoint inhibitor-induced cardiotoxicity and their potential clinical applications.Am J Cancer Res. 2022 Aug 15;12(8):3548-3560. eCollection 2022. Am J Cancer Res. 2022. PMID: 36119829 Free PMC article. Review.
-
Multimodality Advanced Cardiovascular and Molecular Imaging for Early Detection and Monitoring of Cancer Therapy-Associated Cardiotoxicity and the Role of Artificial Intelligence and Big Data.Front Cardiovasc Med. 2022 Mar 15;9:829553. doi: 10.3389/fcvm.2022.829553. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35369354 Free PMC article. Review.
-
Utilizing Immuno-Oncology registry data for enhanced non-small cell lung cancer treatment predictions.JAMIA Open. 2025 Jul 9;8(4):ooaf069. doi: 10.1093/jamiaopen/ooaf069. eCollection 2025 Aug. JAMIA Open. 2025. PMID: 40636413 Free PMC article.
-
The artificial intelligence and machine learning in lung cancer immunotherapy.J Hematol Oncol. 2023 May 24;16(1):55. doi: 10.1186/s13045-023-01456-y. J Hematol Oncol. 2023. PMID: 37226190 Free PMC article. Review.
-
Cardiovascular Toxicity Associated With Immune Checkpoint Inhibitors: Interpreting the Discrepancy Between Clinical Trials and Real-World Data.Cureus. 2025 Jun 30;17(6):e87049. doi: 10.7759/cureus.87049. eCollection 2025 Jun. Cureus. 2025. PMID: 40741581 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
