Adoptive cell therapy with tumor-infiltrating lymphocytes supported by checkpoint inhibition across multiple solid cancer types
- PMID: 34607899
- PMCID: PMC8491427
- DOI: 10.1136/jitc-2021-003499
Adoptive cell therapy with tumor-infiltrating lymphocytes supported by checkpoint inhibition across multiple solid cancer types
Abstract
Background: Adoptive cell therapy (ACT) with tumor-infiltrating lymphocytes (TILs) has shown remarkable results in malignant melanoma (MM), while studies on the potential in other cancer diagnoses are sparse. Further, the prospect of using checkpoint inhibitors (CPIs) to support TIL production and therapy remains to be explored.
Study design: TIL-based ACT with CPIs was evaluated in a clinical phase I/II trial. Ipilimumab (3 mg/kg) was administered prior to tumor resection and nivolumab (3 mg/kg, every 2 weeks ×4) in relation to TIL infusion. Preconditioning chemotherapy was given before TIL infusion and followed by low-dose (2 10e6 international units (UI) ×1 subcutaneous for 14 days) interleukin-2 stimulation.
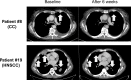
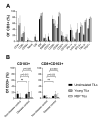
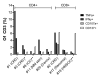
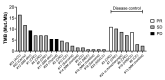
Results: Twenty-five patients covering 10 different cancer diagnoses were treated with in vitro expanded TILs. Expansion of TILs was successful in 97% of recruited patients. Five patients had sizeable tumor regressions of 30%-63%, including two confirmed partial responses in patients with head-and-neck cancer and cholangiocarcinoma. Safety and feasibility were comparable to MM trials of ACT with the addition of expected CPI toxicity. In an exploratory analysis, tumor mutational burden and expression of the alpha-integrin CD103 (p=0.025) were associated with increased disease control. In vitro tumor reactivity was seen in both patients with an objective response and was associated with regressions in tumor size (p=0.028).
Conclusion: High success rates of TIL expansion were demonstrated across multiple solid cancers. TIL ACTs were found feasible, independent of previous therapy. Tumor regressions after ACT combined with CPIs were demonstrated in several cancer types supported by in vitro antitumor reactivity of the TILs.
Trial registration numbers: NCT03296137, and EudraCT No. 2017-002323-25.
Keywords: adoptive; immunotherapy; lymphocytes; tumor-infiltrating.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures


Similar articles
-
Adoptive cell therapy using tumor-infiltrating lymphocytes for melanoma refractory to immune-checkpoint inhibitors.Cancer Sci. 2021 Aug;112(8):3163-3172. doi: 10.1111/cas.15009. Epub 2021 Jun 30. Cancer Sci. 2021. PMID: 34101300 Free PMC article. Clinical Trial.
-
Combination Nivolumab, CD137 Agonism, and Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes for Patients with Metastatic Melanoma.Clin Cancer Res. 2022 Dec 15;28(24):5317-5329. doi: 10.1158/1078-0432.CCR-22-2103. Clin Cancer Res. 2022. PMID: 36215121 Free PMC article. Clinical Trial.
-
Phase II clinical trial of adoptive cell therapy for patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and low-dose interleukin-2.Cancer Immunol Immunother. 2019 May;68(5):773-785. doi: 10.1007/s00262-019-02307-x. Epub 2019 Feb 11. Cancer Immunol Immunother. 2019. PMID: 30747243 Free PMC article. Clinical Trial.
-
Optimizing TIL therapy for uveal melanoma: lessons learned and unlearned from cutaneous melanoma.Immunotherapy. 2025 Mar;17(4):283-291. doi: 10.1080/1750743X.2025.2478808. Epub 2025 Mar 18. Immunotherapy. 2025. PMID: 40098478 Free PMC article. Review.
-
Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option.J Immunother Cancer. 2018 Oct 3;6(1):102. doi: 10.1186/s40425-018-0391-1. J Immunother Cancer. 2018. PMID: 30285902 Free PMC article. Review.
Cited by
-
BiTE secretion by adoptively transferred stem-like T cells improves FRα+ ovarian cancer control.J Immunother Cancer. 2023 Jun;11(6):e006863. doi: 10.1136/jitc-2023-006863. Epub 2023 Jun 23. J Immunother Cancer. 2023. PMID: 37647218 Free PMC article.
-
Individualized Treatment Strategy for Cutaneous Melanoma: Where Are We Now and Where Are We Going?Front Oncol. 2021 Nov 4;11:775100. doi: 10.3389/fonc.2021.775100. eCollection 2021. Front Oncol. 2021. PMID: 34804979 Free PMC article. Review.
-
Single cell analysis in head and neck cancer reveals potential immune evasion mechanisms during early metastasis.Nat Commun. 2023 Mar 27;14(1):1680. doi: 10.1038/s41467-023-37379-y. Nat Commun. 2023. PMID: 36973261 Free PMC article.
-
Neoantigens: promising targets for cancer therapy.Signal Transduct Target Ther. 2023 Jan 6;8(1):9. doi: 10.1038/s41392-022-01270-x. Signal Transduct Target Ther. 2023. PMID: 36604431 Free PMC article. Review.
-
Investigating the Potential of Isolating and Expanding Tumour-Infiltrating Lymphocytes from Adult Sarcoma.Cancers (Basel). 2022 Jan 21;14(3):548. doi: 10.3390/cancers14030548. Cancers (Basel). 2022. PMID: 35158816 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
