Eicosanoid regulation of debris-stimulated metastasis
- PMID: 34607951
- PMCID: PMC8521662
- DOI: 10.1073/pnas.2107771118
Eicosanoid regulation of debris-stimulated metastasis
Abstract
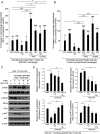
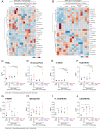
Cancer therapy reduces tumor burden via tumor cell death ("debris"), which can accelerate tumor progression via the failure of inflammation resolution. Thus, there is an urgent need to develop treatment modalities that stimulate the clearance or resolution of inflammation-associated debris. Here, we demonstrate that chemotherapy-generated debris stimulates metastasis by up-regulating soluble epoxide hydrolase (sEH) and the prostaglandin E2 receptor 4 (EP4). Therapy-induced tumor cell debris triggers a storm of proinflammatory and proangiogenic eicosanoid-driven cytokines. Thus, targeting a single eicosanoid or cytokine is unlikely to prevent chemotherapy-induced metastasis. Pharmacological abrogation of both sEH and EP4 eicosanoid pathways prevents hepato-pancreatic tumor growth and liver metastasis by promoting macrophage phagocytosis of debris and counterregulating a protumorigenic eicosanoid and cytokine storm. Therefore, stimulating the clearance of tumor cell debris via combined sEH and EP4 inhibition is an approach to prevent debris-stimulated metastasis and tumor growth.
Keywords: autacoid; debris; inflammation resolution; prostaglandin E2 receptor 4; soluble epoxide hydrolase.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: Y.S., K.-K.H., X.Y., and B.D.H. work with Ionova and EicOsis, respectively, on the development of EP4 and epoxide hydrolase inhibitors for clinical use. D.P., A.G., and B.D.H., and colleagues C. N. Serhan and P. Sime were coauthors on an earlier commentary suggesting modulating the eicosanoid and cytokine storms with a soluble epoxide hydrolase inhibitor to modulate severity of COVID infections, introducing the same mechanism as is suggested here for reduction of the cytokine storm following cancer therapy.
Figures




Similar articles
-
Resolution of eicosanoid/cytokine storm prevents carcinogen and inflammation-initiated hepatocellular cancer progression.Proc Natl Acad Sci U S A. 2020 Sep 1;117(35):21576-21587. doi: 10.1073/pnas.2007412117. Epub 2020 Aug 14. Proc Natl Acad Sci U S A. 2020. PMID: 32801214 Free PMC article.
-
Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor.Proc Natl Acad Sci U S A. 2019 Jan 29;116(5):1698-1703. doi: 10.1073/pnas.1803999116. Epub 2019 Jan 15. Proc Natl Acad Sci U S A. 2019. PMID: 30647111 Free PMC article.
-
Debris-stimulated tumor growth: a Pandora's box?Cancer Metastasis Rev. 2021 Sep;40(3):791-801. doi: 10.1007/s10555-021-09998-8. Epub 2021 Oct 19. Cancer Metastasis Rev. 2021. PMID: 34665387 Free PMC article. Review.
-
Enhancing cancer immunotherapy via inhibition of soluble epoxide hydrolase.Proc Natl Acad Sci U S A. 2024 Feb 13;121(7):e2314085121. doi: 10.1073/pnas.2314085121. Epub 2024 Feb 8. Proc Natl Acad Sci U S A. 2024. PMID: 38330013 Free PMC article.
-
Carcinogenesis: Failure of resolution of inflammation?Pharmacol Ther. 2021 Feb;218:107670. doi: 10.1016/j.pharmthera.2020.107670. Epub 2020 Sep 3. Pharmacol Ther. 2021. PMID: 32891711 Free PMC article. Review.
Cited by
-
Dual Blockade of EP2 and EP4 Signaling is Required for Optimal Immune Activation and Antitumor Activity Against Prostaglandin-Expressing Tumors.Cancer Res Commun. 2023 Aug 8;3(8):1486-1500. doi: 10.1158/2767-9764.CRC-23-0249. eCollection 2023 Aug. Cancer Res Commun. 2023. PMID: 37559947 Free PMC article.
-
Macrophage Inactivation by Small Molecule Wedelolactone via Targeting sEH for the Treatment of LPS-Induced Acute Lung Injury.ACS Cent Sci. 2023 Feb 21;9(3):440-456. doi: 10.1021/acscentsci.2c01424. eCollection 2023 Mar 22. ACS Cent Sci. 2023. PMID: 36968547 Free PMC article.
-
Minimally invasive biopsy-based diagnostics in support of precision cancer medicine.Mol Oncol. 2024 Nov;18(11):2612-2628. doi: 10.1002/1878-0261.13640. Epub 2024 Mar 22. Mol Oncol. 2024. PMID: 38519839 Free PMC article. Review.
-
Investigating the role of the mPGES-PGE₂-EP4 pathway in Escherichia coli-induced mastitis in dairy cows: insights for non-antibiotic therapeutic strategies.Front Vet Sci. 2025 Jul 1;12:1628028. doi: 10.3389/fvets.2025.1628028. eCollection 2025. Front Vet Sci. 2025. PMID: 40666730 Free PMC article.
-
Inhibition of Soluble Epoxide Hydrolase Reduces Inflammation and Myocardial Injury in Arrhythmogenic Cardiomyopathy.JACC Basic Transl Sci. 2025 Mar;10(3):367-380. doi: 10.1016/j.jacbts.2024.12.010. Epub 2025 Feb 5. JACC Basic Transl Sci. 2025. PMID: 40139877 Free PMC article.

