Tubular lysosomes harbor active ion gradients and poise macrophages for phagocytosis
- PMID: 34607961
- PMCID: PMC8522270
- DOI: 10.1073/pnas.2113174118
Tubular lysosomes harbor active ion gradients and poise macrophages for phagocytosis
Abstract
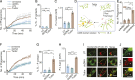
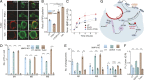
Lysosomes adopt dynamic, tubular states that regulate antigen presentation, phagosome resolution, and autophagy. Tubular lysosomes are studied either by inducing autophagy or by activating immune cells, both of which lead to cell states where lysosomal gene expression differs from the resting state. Therefore, it has been challenging to pinpoint the biochemical properties lysosomes acquire upon tubulation that could drive their functionality. Here we describe a DNA-based assembly that tubulates lysosomes in macrophages without activating them. Proteolytic activity maps at single-lysosome resolution revealed that tubular lysosomes were less degradative and showed proximal to distal luminal pH and Ca2+ gradients. Such gradients had been predicted but never previously observed. We identify a role for tubular lysosomes in promoting phagocytosis and activating MMP9. The ability to tubulate lysosomes without starving or activating immune cells may help reveal new roles for tubular lysosomes.
Keywords: DNA nanotechnology; MMP9; lysosomes; macrophages; phagocytosis.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
Phagosome resolution regenerates lysosomes and maintains the degradative capacity in phagocytes.J Cell Biol. 2021 Sep 6;220(9):e202005072. doi: 10.1083/jcb.202005072. Epub 2021 Jun 28. J Cell Biol. 2021. PMID: 34180943 Free PMC article.
-
The Phosphoinositide-Gated Lysosomal Ca(2+) Channel, TRPML1, Is Required for Phagosome Maturation.Traffic. 2015 Sep;16(9):1010-26. doi: 10.1111/tra.12303. Epub 2015 Jun 18. Traffic. 2015. PMID: 26010303
-
Phagocytosis Enhances Lysosomal and Bactericidal Properties by Activating the Transcription Factor TFEB.Curr Biol. 2016 Aug 8;26(15):1955-1964. doi: 10.1016/j.cub.2016.05.070. Epub 2016 Jul 7. Curr Biol. 2016. PMID: 27397893 Free PMC article.
-
Autophagy proteins regulate cell engulfment mechanisms that participate in cancer.Semin Cancer Biol. 2013 Oct;23(5):329-36. doi: 10.1016/j.semcancer.2013.05.004. Epub 2013 May 30. Semin Cancer Biol. 2013. PMID: 23726896 Free PMC article. Review.
-
Choreographing endo-lysosomal Ca2+ throughout the life of a phagosome.Biochim Biophys Acta Mol Cell Res. 2021 Jun;1868(7):119040. doi: 10.1016/j.bbamcr.2021.119040. Epub 2021 Apr 17. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 33872669 Review.
Cited by
-
Kinetics of phagosome maturation is coupled to their intracellular motility.Commun Biol. 2022 Sep 26;5(1):1014. doi: 10.1038/s42003-022-03988-4. Commun Biol. 2022. PMID: 36163370 Free PMC article.
-
Exploring the Subcellular Localization and Degradation of Spherical Nucleic Acids Using Fluorescence Lifetime Imaging Microscopy.ACS Nano. 2025 Jun 24;19(24):21983-21996. doi: 10.1021/acsnano.5c00177. Epub 2025 Jun 9. ACS Nano. 2025. PMID: 40489247 Free PMC article.
-
Detection and quantification of the vacuolar H+ATPase using the Legionella effector protein SidK.J Cell Biol. 2022 Mar 7;221(3):e202107174. doi: 10.1083/jcb.202107174. Epub 2022 Jan 13. J Cell Biol. 2022. PMID: 35024770 Free PMC article.
-
The ion channels of endomembranes.Physiol Rev. 2024 Jul 1;104(3):1335-1385. doi: 10.1152/physrev.00025.2023. Epub 2024 Mar 7. Physiol Rev. 2024. PMID: 38451235 Free PMC article. Review.
-
The evolution of organellar calcium mapping technologies.Cell Calcium. 2022 Dec;108:102658. doi: 10.1016/j.ceca.2022.102658. Epub 2022 Oct 11. Cell Calcium. 2022. PMID: 36274564 Free PMC article. Review.
References
-
- Hipolito V. E. B., Ospina-Escobar E., Botelho R. J., Lysosome remodelling and adaptation during phagocyte activation. Cell. Microbiol. 20, e12824 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

