Leu-to-Phe substitution at prM146 decreases the growth ability of Zika virus and partially reduces its pathogenicity in mice
- PMID: 34608212
- PMCID: PMC8490429
- DOI: 10.1038/s41598-021-99086-2
Leu-to-Phe substitution at prM146 decreases the growth ability of Zika virus and partially reduces its pathogenicity in mice
Abstract
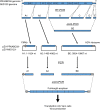
Zika virus (ZIKV) is a mosquito-borne flavivirus that causes febrile illness. The recent spread of ZIKV from Asia to the Americas via the Pacific region has revealed unprecedented features of ZIKV, including transplacental congenital infection causing microcephaly. Amino acid changes have been hypothesized to underlie the spread and novel features of American ZIKV strains; however, the relationship between genetic changes and the epidemic remains controversial. A comparison of the characteristics of a Southeast Asian strain (NIID123) and an American strain (PRVABC59) revealed that the latter had a higher replication ability in cultured cells and higher virulence in mice. In this study, we aimed to identify the genetic region of ZIKV responsible for these different characteristics using reverse genetics. A chimeric NIID123 strain in which the E protein was replaced with that of PRVABC59 showed a lower growth ability than the recombinant wild-type strain. Adaptation of the chimeric NIID123 to Vero cells induced a Phe-to-Leu amino acid substitution at position 146 of the prM protein; PRVABC59 also has Leu at this position. Leu at this position was found to be responsible for the viral replication ability and partially, for the pathogenicity in mouse testes.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

