Determining the immune environment of cutaneous T-cell lymphoma lesions through the assessment of lesional blood drops
- PMID: 34608214
- PMCID: PMC8490448
- DOI: 10.1038/s41598-021-98804-0
Determining the immune environment of cutaneous T-cell lymphoma lesions through the assessment of lesional blood drops
Abstract
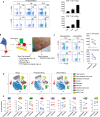
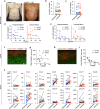
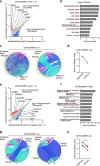
Detailed analysis of the cells that infiltrate lesional skin cannot be performed in skin biopsy specimens using immunohistochemistry or cell separation techniques because enzyme treatments applied during the isolation step can destroy small amounts of protein and minor cell populations in the biopsy specimen. Here, we describe a method for isolating T cells from drops of whole blood obtained from lesions during skin biopsy in patients with cutaneous T-cell lymphoma. Lesional blood is assumed to contain lesional resident cells, cells from capillary vessels, and blood overflowing from capillary vessels into the lesion area. The lesional blood showed substantial increases in distinct cell populations, chemokines, and the expression of various genes. The proportion of CD8+CD45RO+ T cells in the lesional blood negatively correlated with the modified severity-weighted assessment tool scores. CD4+CD45RO+ T cells in the lesional blood expressed genes associated with the development of cancer and progression of cutaneous T-cell lymphoma. In addition, CD8+CD45RO+ T cells in lesional blood had unique T-cell receptor repertoires in lesions of each stage. Assessment of lesional blood drops might provide new insight into the pathogenesis of mycosis fungoides and facilitate evaluation of the treatment efficacy for mycosis fungoides as well as other skin inflammatory diseases.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Rare Cutaneous T-Cell Lymphomas.Hematol Oncol Clin North Am. 2019 Feb;33(1):135-148. doi: 10.1016/j.hoc.2018.08.004. Hematol Oncol Clin North Am. 2019. PMID: 30497671 Review.
-
CD4+CD26- lymphocytes are useful to assess blood involvement and define B ratings in cutaneous T cell lymphoma.Leuk Lymphoma. 2018 Feb;59(2):330-339. doi: 10.1080/10428194.2017.1334123. Epub 2017 Jun 9. Leuk Lymphoma. 2018. PMID: 28595473
-
Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma.Oncotarget. 2017 Jul 4;8(27):44418-44433. doi: 10.18632/oncotarget.17901. Oncotarget. 2017. PMID: 28574843 Free PMC article.
-
Increased HMGB1 levels in lesional skin and sera in patients with cutaneous T-cell lymphoma.Eur J Dermatol. 2018 Oct 1;28(5):621-627. doi: 10.1684/ejd.2018.3400. Eur J Dermatol. 2018. PMID: 30378541
-
Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers.J Am Acad Dermatol. 2014 Feb;70(2):205.e1-16; quiz 221-2. doi: 10.1016/j.jaad.2013.07.049. J Am Acad Dermatol. 2014. PMID: 24438969 Review.
Cited by
-
A case of mycosis fungoides effectively treated with ultraviolet light A 1-light-emitting diode phototherapy.Photodermatol Photoimmunol Photomed. 2023 Jan;39(1):69-71. doi: 10.1111/phpp.12798. Epub 2022 May 31. Photodermatol Photoimmunol Photomed. 2023. PMID: 35527358 Free PMC article. No abstract available.
References
-
- Bodenlenz M, et al. Clinical applicability of dOFM devices for dermal sampling. Skin Res. Technol. 2013;19:474–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

