RNA binding protein RBMS3 is a common EMT effector that modulates triple-negative breast cancer progression via stabilizing PRRX1 mRNA
- PMID: 34608266
- PMCID: PMC9421946
- DOI: 10.1038/s41388-021-02030-x
RNA binding protein RBMS3 is a common EMT effector that modulates triple-negative breast cancer progression via stabilizing PRRX1 mRNA
Abstract
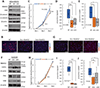
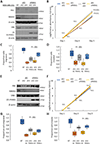
The epithelial-to-mesenchymal transition (EMT) has been recognized as a driving force for tumor progression in breast cancer. Recently, our group identified the RNA Binding Motif Single Stranded Interacting Protein 3 (RBMS3) to be significantly associated with an EMT transcriptional program in breast cancer. Additional expression profiling demonstrated that RBMS3 was consistently upregulated by multiple EMT transcription factors and correlated with mesenchymal gene expression in breast cancer cell lines. Functionally, RBMS3 was sufficient to induce EMT in two immortalized mammary epithelial cell lines. In triple-negative breast cancer (TNBC) models, RBMS3 was necessary for maintaining the mesenchymal phenotype and invasion and migration in vitro. Loss of RBMS3 significantly impaired both tumor progression and spontaneous metastasis in vivo. Using a genome-wide approach to interrogate mRNA stability, we found that ectopic expression of RBMS3 upregulates many genes that are resistant to degradation following transcriptional blockade by actinomycin D (ACTD). Specifically, RBMS3 was shown to interact with the mRNA of EMT transcription factor PRRX1 and promote PRRX1 mRNA stability. PRRX1 is required for RBMS3-mediated EMT and is partially sufficient to rescue the effect of RBMS3 knockdown in TNBC cell lines. Together, this study identifies RBMS3 as a novel and common effector of EMT, which could be a promising therapeutic target for TNBC treatment.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures





References
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. - PubMed
-
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

