The EGFRvIII transcriptome in glioblastoma: A meta-omics analysis
- PMID: 34608482
- PMCID: PMC8917407
- DOI: 10.1093/neuonc/noab231
The EGFRvIII transcriptome in glioblastoma: A meta-omics analysis
Abstract
Background: EGFR is among the genes most frequently altered in glioblastoma, with exons 2-7 deletions (EGFRvIII) being among its most common genomic mutations. There are conflicting reports about its prognostic role and it remains unclear whether and how it differs in signaling compared with wildtype EGFR.
Methods: To better understand the oncogenic role of EGFRvIII, we leveraged 4 large datasets into 1 large glioblastoma transcriptome dataset (n = 741) alongside 81 whole-genome samples from 2 datasets.
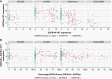
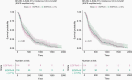
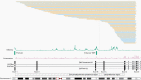
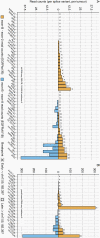
Results: The EGFRvIII/EGFR expression ratios differ strongly between tumors and range from 1% to 95%. Interestingly, the slope of relative EGFRvIII expression is near-linear, which argues against a more positive selection pressure than EGFR wildtype. An absence of selection pressure is also suggested by the similar survival between EGFRvIII-positive and -negative glioblastoma patients. EGFRvIII levels are inversely correlated with pan-EGFR (all wildtype and mutant variants) expression, which indicates that EGFRvIII has a higher potency in downstream pathway activation. EGFRvIII-positive glioblastomas have a lower CDK4 or MDM2 amplification incidence than EGFRvIII-negative (P = .007), which may point toward crosstalk between these pathways. EGFRvIII-expressing tumors have an upregulation of "classical" subtype genes compared to those with EGFR-amplification only (P = 3.873e-6). Genomic breakpoints of the EGFRvIII deletions have a preference toward the 3'-end of the large intron-1. These preferred breakpoints preserve a cryptic exon resulting in a novel EGFRvIII variant and preserve an intronic enhancer.
Conclusions: These data provide deeper insights into the complex EGFRvIII biology and provide new insights for targeting EGFRvIII mutated tumors.
Keywords: EGFR; EGFRvIII; RNA-seq; breakpoints; glioblastoma.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Figures


References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

