Spinal cord stimulation reduces cardiac pain through microglial deactivation in rats with chronic myocardial ischemia
- PMID: 34608504
- PMCID: PMC8503748
- DOI: 10.3892/mmr.2021.12475
Spinal cord stimulation reduces cardiac pain through microglial deactivation in rats with chronic myocardial ischemia
Abstract
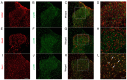
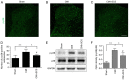
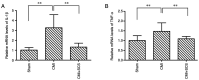
Angina pectoris is cardiac pain that is a common clinical symptom often resulting from myocardial ischemia. Spinal cord stimulation (SCS) is effective in treating refractory angina pectoris, but its underlying mechanisms have not been fully elucidated. The spinal dorsal horn is the first region of the central nervous system that receives nociceptive information; it is also the target of SCS. In the spinal cord, glial (astrocytes and microglia) activation is involved in the initiation and persistence of chronic pain. Thus, the present study investigated the possible cardiac pain‑relieving effects of SCS on spinal dorsal horn glia in chronic myocardial ischemia (CMI). CMI was established by left anterior descending artery ligation surgery, which induced significant spontaneous/ongoing cardiac pain behaviors, as measured using the open field test in rats. SCS effectively improved such behaviors as shown by open field and conditioned place preference tests in CMI model rats. SCS suppressed CMI‑induced spinal dorsal horn microglial activation, with downregulation of ionized calcium‑binding adaptor protein‑1 expression. Moreover, SCS inhibited CMI‑induced spinal expression of phosphorylated‑p38 MAPK, which was specifically colocalized with the spinal dorsal horn microglia rather than astrocytes and neurons. Furthermore, SCS could depress spinal neuroinflammation by suppressing CMI‑induced IL‑1β and TNF‑α release. Intrathecal administration of minocycline, a microglial inhibitor, alleviated the cardiac pain behaviors in CMI model rats. In addition, the injection of fractalkine (microglia‑activating factor) partially reversed the SCS‑produced analgesic effects on CMI‑induced cardiac pain. These results indicated that the therapeutic mechanism of SCS on CMI may occur partially through the inhibition of spinal microglial p38 MAPK pathway activation. The present study identified a novel mechanism underlying the SCS‑produced analgesic effects on chronic cardiac pain.
Keywords: cardiac pain; chronic myocardial ischemia; microglia; spinal cord stimulation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Spinal Cord Stimulation Alleviates Neuropathic Pain by Attenuating Microglial Activation via Reducing Colony-Stimulating Factor 1 Levels in the Spinal Cord in a Rat Model of Chronic Constriction Injury.Anesth Analg. 2022 Jul 1;135(1):178-190. doi: 10.1213/ANE.0000000000006016. Epub 2022 Apr 4. Anesth Analg. 2022. PMID: 35709447 Free PMC article.
-
Alendronate Attenuates Spinal Microglial Activation and Neuropathic Pain.J Pain. 2016 Aug;17(8):889-903. doi: 10.1016/j.jpain.2016.03.008. Epub 2016 Apr 6. J Pain. 2016. PMID: 27063783
-
Interaction between astrocytic colony stimulating factor and its receptor on microglia mediates central sensitization and behavioral hypersensitivity in chronic post ischemic pain model.Brain Behav Immun. 2018 Feb;68:248-260. doi: 10.1016/j.bbi.2017.10.023. Epub 2017 Oct 31. Brain Behav Immun. 2018. PMID: 29080683
-
Microglia: a promising target for treating neuropathic and postoperative pain, and morphine tolerance.J Formos Med Assoc. 2011 Aug;110(8):487-94. doi: 10.1016/S0929-6646(11)60074-0. J Formos Med Assoc. 2011. PMID: 21783017 Free PMC article. Review.
-
Mechanisms of spinal cord stimulation for the treatment of pain: Still in the dark after 50 years.Eur J Pain. 2019 Apr;23(4):652-659. doi: 10.1002/ejp.1336. Epub 2018 Dec 3. Eur J Pain. 2019. PMID: 30407696 Free PMC article. Review.
Cited by
-
Improvement of brain perfusion in patients with chronic brain ischemia at epidural spinal cord electrical stimulation.Front Surg. 2022 Sep 23;9:1026079. doi: 10.3389/fsurg.2022.1026079. eCollection 2022. Front Surg. 2022. PMID: 36211284 Free PMC article.
-
Spinal Mechanisms of Pain Modulation by Spinal Cord Stimulation: A Systematic Review.Cureus. 2025 Jun 8;17(6):e85567. doi: 10.7759/cureus.85567. eCollection 2025 Jun. Cureus. 2025. PMID: 40636634 Free PMC article. Review.
-
Artificial Intelligence-Guided Neuromodulation in Heart Failure with Preserved and Reduced Ejection Fraction: Mechanisms, Evidence, and Future Directions.J Cardiovasc Dev Dis. 2025 Aug 19;12(8):314. doi: 10.3390/jcdd12080314. J Cardiovasc Dev Dis. 2025. PMID: 40863380 Free PMC article. Review.
-
Spinal cord stimulation for refractory pericarditis: a case report and a review of the mechanism of action.Front Pain Res (Lausanne). 2023 Jul 5;4:1174044. doi: 10.3389/fpain.2023.1174044. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 37476333 Free PMC article.
-
Pain in the body, harm to the heart: advances in research on the impact of chronic pain on cardiovascular diseases.Front Cardiovasc Med. 2025 Aug 6;12:1629145. doi: 10.3389/fcvm.2025.1629145. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40842473 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

