WNT3 hypomethylation counteracts low activity of the Wnt signaling pathway in the placenta of preeclampsia
- PMID: 34608506
- PMCID: PMC8558176
- DOI: 10.1007/s00018-021-03941-4
WNT3 hypomethylation counteracts low activity of the Wnt signaling pathway in the placenta of preeclampsia
Abstract
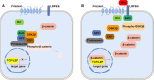
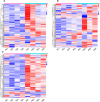
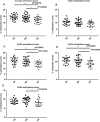
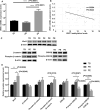
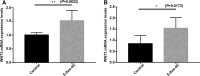
Preeclampsia is a hypertensive disorder of pregnancy. Many studies have shown that epigenetic mechanisms may play a role in preeclampsia. Moreover, our previous study indicated that the differentially methylated genes in preeclampsia were enriched in the Wnt/β-catenin signaling pathway. This study aimed to identify differentially methylated Wnt/β-catenin signaling pathway genes in the preeclamptic placenta and to study the roles of these genes in trophoblast cells in vitro. Using an Illumina Infinium HumanMethylation 850 K BeadChip, we found that the Wnt signaling pathway was globally hypermethylated in the preeclamptic group compared with the term birth group, but hypomethylated in the preeclamptic group compared with the preterm birth group. Among all Wnt/β-catenin signaling pathway factors, WNT3 was the most significantly differentially expressed gene and was hypomethylated in the preeclamptic group compared to the nonhypertensive groups, namely, the preterm birth group and term birth group. This result was confirmed by pyrosequencing. Through quantitative real-time PCR and western blot analysis, the WNT3 gene was found to be highly expressed in preeclamptic placental tissues, in contrast to other WNT factors, which were previously reported to be expressed at low levels in placental tissues. Additionally, in the HTR8/SVneo cell line, knockdown of WNT3 suppressed the Wnt/β-catenin signaling pathway, consistent with the findings for other WNT factors. These results prompted us to speculate that the WNT3 gene counteracts the low activation state of the Wnt signaling pathway in the preeclamptic placenta through methylation modification.
Keywords: DNA methylation; Placentas; Preeclampsia; Preterm birth; WNT3 gene.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


Similar articles
-
[Expression and significance of SATB1 and wnt/β-catenin signaling molecule in the placenta of preeclampsia].Zhonghua Fu Chan Ke Za Zhi. 2015 Apr;50(4):283-90. Zhonghua Fu Chan Ke Za Zhi. 2015. PMID: 26080941 Chinese.
-
Differential placental methylation in preeclampsia, preterm and term pregnancies.Placenta. 2020 Apr;93:56-63. doi: 10.1016/j.placenta.2020.02.009. Epub 2020 Feb 11. Placenta. 2020. PMID: 32250740
-
DNA methylation profiles in preeclampsia and healthy control placentas.Am J Physiol Heart Circ Physiol. 2016 May 15;310(10):H1295-303. doi: 10.1152/ajpheart.00958.2015. Epub 2016 Mar 11. Am J Physiol Heart Circ Physiol. 2016. PMID: 26968548
-
Wnt/β-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia (Review).Mol Med Rep. 2017 Aug;16(2):1007-1013. doi: 10.3892/mmr.2017.6718. Epub 2017 Jun 7. Mol Med Rep. 2017. PMID: 29067442 Review.
-
Dysregulation of LncRNAs in Placenta and Pathogenesis of Preeclampsia.Curr Drug Targets. 2017;18(10):1165-1170. doi: 10.2174/1389450118666170404160000. Curr Drug Targets. 2017. PMID: 28382860 Review.
Cited by
-
Downregulation of CMIP contributes to preeclampsia development by impairing trophoblast function via the PDE7B-cAMP pathway.Cell Mol Life Sci. 2025 May 15;82(1):203. doi: 10.1007/s00018-025-05726-5. Cell Mol Life Sci. 2025. PMID: 40372501 Free PMC article.
-
Basic Research Advances in China on Embryo Implantation, Placentation, and Parturition.Matern Fetal Med. 2024 Jan 15;6(1):37-49. doi: 10.1097/FM9.0000000000000210. eCollection 2024 Jan. Matern Fetal Med. 2024. PMID: 40406740 Free PMC article.
-
DNA methylation landscape in pregnancy-induced hypertension: progress and challenges.Reprod Biol Endocrinol. 2024 Jul 8;22(1):77. doi: 10.1186/s12958-024-01248-0. Reprod Biol Endocrinol. 2024. PMID: 38978060 Free PMC article. Review.
-
High-throughput methylome analysis reveals differential methylation for early and late onset preeclampsia for mothers and their children.Physiol Genomics. 2024 Mar 1;56(3):276-282. doi: 10.1152/physiolgenomics.00058.2023. Epub 2024 Jan 8. Physiol Genomics. 2024. PMID: 38189116 Free PMC article.
-
Exosomal encapsulation of miR-3198 promotes proliferation and migration of trophoblasts in preeclampsia.J Assist Reprod Genet. 2024 May;41(5):1403-1416. doi: 10.1007/s10815-024-03104-x. Epub 2024 Mar 27. J Assist Reprod Genet. 2024. PMID: 38536597 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

