Frontal white matter lesions in Alzheimer's disease are associated with both small vessel disease and AD-associated cortical pathology
- PMID: 34608542
- PMCID: PMC8568857
- DOI: 10.1007/s00401-021-02376-2
Frontal white matter lesions in Alzheimer's disease are associated with both small vessel disease and AD-associated cortical pathology
Abstract
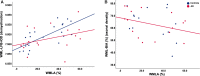
Cerebral white matter lesions (WML) encompass axonal loss and demyelination and are assumed to be associated with small vessel disease (SVD)-related ischaemia. However, our previous study in the parietal lobe white matter revealed that WML in Alzheimer's disease (AD) are linked with degenerative axonal loss secondary to the deposition of cortical AD pathology. Furthermore, neuroimaging data suggest that pathomechanisms for the development of WML differ between anterior and posterior lobes with AD-associated degenerative mechanism driving posterior white matter disruption, and both AD-associated degenerative and vascular mechanisms contributed to anterior matter disruption. In this pilot study, we used human post-mortem brain tissue to investigate the composition and aetiology of frontal WML from AD and non-demented controls to determine if frontal WML are SVD-associated and to reveal any regional differences in the pathogenesis of WML. Frontal WML tissue sections from 40 human post-mortem brains (AD, n = 19; controls, n = 21) were quantitatively assessed for demyelination, axonal loss, cortical hyperphosphorylated tau (HPτ) and amyloid-beta (Aβ) burden, and arteriolosclerosis as a measure of SVD. Biochemical assessment included Wallerian degeneration-associated protease calpain and the myelin-associated glycoprotein to proteolipid protein ratio as a measure of ante-mortem ischaemia. Arteriolosclerosis severity was found to be associated with and a significant predictor of frontal WML severity in both AD and non-demented controls. Interesting, frontal axonal loss was also associated with HPτ and calpain levels were associated with increasing Aβ burden in the AD group, suggestive of an additional degenerative influence. To conclude, this pilot data suggest that frontal WML in AD may result from both increased arteriolosclerosis and AD-associated degenerative changes. These preliminary findings in combination with previously published data tentatively indicate regional differences in the aetiology of WML in AD, which should be considered in the clinical diagnosis of dementia subtypes: posterior WML maybe associated with degenerative mechanisms secondary to AD pathology, while anterior WML could be associated with both SVD-associated and degenerative mechanisms.
Keywords: Alzheimer’s disease; Amyloid-beta; Hyperphosphorylated tau; Small vessel disease; White matter hyperintensity; White matter lesion.
© 2021. Crown.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Parietal white matter lesions in Alzheimer's disease are associated with cortical neurodegenerative pathology, but not with small vessel disease.Acta Neuropathol. 2017 Sep;134(3):459-473. doi: 10.1007/s00401-017-1738-2. Epub 2017 Jun 21. Acta Neuropathol. 2017. PMID: 28638989 Free PMC article.
-
Extravascular fibrinogen in the white matter of Alzheimer's disease and normal aged brains: implications for fibrinogen as a biomarker for Alzheimer's disease.Brain Pathol. 2019 May;29(3):414-424. doi: 10.1111/bpa.12685. Epub 2019 Jan 29. Brain Pathol. 2019. PMID: 30485582 Free PMC article.
-
Cortical tau load is associated with white matter hyperintensities.Acta Neuropathol Commun. 2015 Sep 30;3:60. doi: 10.1186/s40478-015-0240-0. Acta Neuropathol Commun. 2015. PMID: 26419828 Free PMC article.
-
Cerebral Small Vessel Disease in Sporadic and Familial Alzheimer Disease.Am J Pathol. 2021 Nov;191(11):1888-1905. doi: 10.1016/j.ajpath.2021.07.004. Epub 2021 Jul 28. Am J Pathol. 2021. PMID: 34331941 Free PMC article. Review.
-
Cerebral small vessel disease and the risk of Alzheimer's disease: A systematic review.Ageing Res Rev. 2018 Nov;47:41-48. doi: 10.1016/j.arr.2018.06.002. Epub 2018 Jun 26. Ageing Res Rev. 2018. PMID: 29898422
Cited by
-
Pathological angiogenesis was associated with cerebrovascular lesion and neurodegeneration in Alzheimer's disease.Alzheimers Dement. 2025 Feb;21(2):e14521. doi: 10.1002/alz.14521. Epub 2025 Jan 8. Alzheimers Dement. 2025. PMID: 39777972 Free PMC article.
-
Intersecting vulnerabilities: Race, Depression, and White Matter Hyperintensity burden in Aging.medRxiv [Preprint]. 2025 Jul 25:2025.07.24.25332172. doi: 10.1101/2025.07.24.25332172. medRxiv. 2025. PMID: 40778176 Free PMC article. Preprint.
-
Vascular dysfunction in sporadic bvFTD: white matter hyperintensity and peripheral vascular biomarkers.Alzheimers Res Ther. 2024 Apr 5;16(1):72. doi: 10.1186/s13195-024-01422-x. Alzheimers Res Ther. 2024. PMID: 38581060 Free PMC article.
-
White matter hyperintensities and cholinergic degeneration as Lewy body disease.Ann Clin Transl Neurol. 2025 Jan;12(1):97-109. doi: 10.1002/acn3.52257. Epub 2024 Dec 9. Ann Clin Transl Neurol. 2025. PMID: 39654300 Free PMC article.
-
Interpretable deep learning of myelin histopathology in age-related cognitive impairment.Acta Neuropathol Commun. 2022 Sep 21;10(1):131. doi: 10.1186/s40478-022-01425-5. Acta Neuropathol Commun. 2022. PMID: 36127723 Free PMC article.
References
-
- Attems J, Jellinger KA, Lintner F. Alzheimer's disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol. 2005;110:222–231. - PubMed

