Sensitivity of two SARS-CoV-2 variants with spike protein mutations to neutralising antibodies
- PMID: 34608598
- PMCID: PMC8489549
- DOI: 10.1007/s11262-021-01871-8
Sensitivity of two SARS-CoV-2 variants with spike protein mutations to neutralising antibodies
Abstract
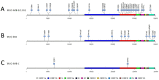
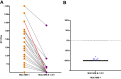
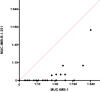
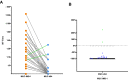
SARS-CoV-2 infections elicit a humoral immune response capable of neutralising the virus. However, multiple variants have emerged with mutations in the spike protein amongst others, the key target of neutralising antibodies. We evaluated the neutralising efficacy of 89 serum samples from patients, infected with SARS-CoV-2 in the beginning of 2020, against two virus variants isolated from acutely infected patients and harbouring spike protein mutations. One isolate was assigned to lineage B.1.351 (MUC-IMB-B.1.351) whilst the other (MUC-484) was isolated from an immunocompromised patient, sharing some but not all mutations with B.1.351 and representing a transitional variant. Both variants showed a significant reduction in neutralisation sensitivity compared to wild-type SARS-CoV-2 with MUC-IMB-B.1.351 being almost completely resistant to neutralisation. The observed reduction in neutralising activity of wild-type-specific antibodies against both variants suggests that individual mutations in the spike protein are sufficient to confer a potent escape from the humoral immune response. In addition, the effect of escape mutations seems to accumulate, so that more heavily mutated variants show a greater loss of sensitivity to neutralisation up to complete insensitivity as observed for MUC-IMB-B.1.351. From a clinical point of view, this might affect the efficacy of (monoclonal) antibody treatment of patients with prolonged infections as well as patients infected with variants other than the donor. At the same, this could also negatively influence the efficacy of current vaccines (as they are based on wild-type spike protein) emphasising the need to thoroughly surveil the emergence and distribution of variants and adapt vaccines and therapeutics accordingly.
Keywords: B.1.351; COVID-19; Immune escape; Mutation E484K; Neutralising antibodies; SARS-CoV-2; Variants of concern.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, Cipolla M, Viant C, Barnes CO, Bram Y, Breton G, Hägglöf T, Mendoza P, Hurley A, Turroja M, Gordon K, Millard KG, Ramos V, Schmidt F, Weisblum Y, Jha D, Tankelevich M, Martinez-Delgado G, Yee J, Patel R, Dizon J, Unson-O’Brien C, Shimeliovich I, Robbiani DF, Zhao Z, Gazumyan A, Schwartz RE, Hatziioannou T, Bjorkman PJ, Mehandru S, Bieniasz PD, Caskey M, Nussenzweig MC. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. - DOI - PMC - PubMed
-
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, COVID-19 Genomics UK (COG-UK) Consortium. Peacock SJ, Robertson DL. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. - DOI - PMC - PubMed
-
- Khatamzas E, Rehn A, Muenchhoff M, Hellmuth J, Gaitzsch E, Weiglein T, Georgi E, Scherer C, Stecher S, Weigert O, Girl P, Zange S, Keppler OT, Stemmler J, Bergwelt-Baildon M von, Wölfel R, Antwerpen M (2021) Emergence of multiple SARS-CoV-2 mutations in an immunocompromised host. medRxiv 2021.01.10.20248871. 10.1101/2021.01.10.20248871
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

