Podoplanin promotes tumor growth, platelet aggregation, and venous thrombosis in murine models of ovarian cancer
- PMID: 34608736
- PMCID: PMC8712373
- DOI: 10.1111/jth.15544
Podoplanin promotes tumor growth, platelet aggregation, and venous thrombosis in murine models of ovarian cancer
Abstract
Background: Podoplanin (PDPN) is a sialylated membrane glycoprotein that binds to C-type lectin-like receptor 2 on platelets resulting in platelet activation. PDPN is expressed on lymphatic endothelial cells, perivascular fibroblasts/pericytes, cancer cells, cancer-associated fibroblasts, and tumor stromal cells. PDPN's expression on malignant epithelial cells plays a role in metastasis. Furthermore, the expression of PDPN in brain tumors (high-grade gliomas) was found to correlate with an increased risk of venous thrombosis.
Objective: We examined the expression of PDPN and its role in tumor progression and venous thrombosis in ovarian cancer.
Methods: We used mouse models of ovarian cancer and venous thrombosis.
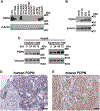
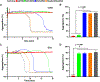
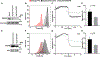
Results: Ovarian cancer cells express PDPN and release PDPN-rich extracellular vesicles (EVs), and cisplatin and topotecan (chemotherapies commonly used in ovarian cancer) increase the expression of podoplanin in cancer cells. The expression of PDPN in ovarian cancer cells promotes tumor growth in a murine model of ovarian cancer and that knockdown of PDPN gene expression results in smaller primary tumors. Both PDPN-expressing ovarian cancer cells and their EVs cause platelet aggregation. In a mouse model of venous thrombosis, PDPN-expressing EVs released from HeyA8 ovarian cancer cells produce more frequent thrombosis than PDPN-negative EVs derived from PDPN-knockdown HeyA8 cells. Blood clots induced by PDPN-positive EVs contain more platelets than those in blood clots induced by PDPN-negative EVs.
Conclusions: In summary, our findings demonstrate that the expression of PDPN by ovarian cancer cells promotes tumor growth and venous thrombosis in mice.
Keywords: murine models of cancer; ovarian cancer; platelet; podoplanin; venous thrombosis.
© 2021 International Society on Thrombosis and Haemostasis.
Figures


Similar articles
-
Intratumoral platelet aggregate formation in a murine preclinical glioma model depends on podoplanin expression on tumor cells.Blood Adv. 2019 Apr 9;3(7):1092-1102. doi: 10.1182/bloodadvances.2018015966. Blood Adv. 2019. PMID: 30948364 Free PMC article.
-
Cancer-associated fibroblasts promote venous thrombosis through podoplanin/CLEC-2 interaction in podoplanin-negative lung cancer mouse model.J Thromb Haemost. 2023 Nov;21(11):3153-3165. doi: 10.1016/j.jtha.2023.07.005. Epub 2023 Jul 18. J Thromb Haemost. 2023. PMID: 37473844
-
Podoplanin promotes cancer-associated thrombosis and contributes to the unfavorable overall survival in an ectopic xenograft mouse model of oral cancer.Biomed J. 2020 Apr;43(2):146-162. doi: 10.1016/j.bj.2019.07.001. Epub 2019 Dec 24. Biomed J. 2020. PMID: 32441651 Free PMC article.
-
Role of Podoplanin (PDPN) in Advancing the Progression and Metastasis of Glioblastoma Multiforme (GBM).Cancers (Basel). 2024 Dec 3;16(23):4051. doi: 10.3390/cancers16234051. Cancers (Basel). 2024. PMID: 39682237 Free PMC article. Review.
-
Roles of Podoplanin in Malignant Progression of Tumor.Cells. 2022 Feb 7;11(3):575. doi: 10.3390/cells11030575. Cells. 2022. PMID: 35159384 Free PMC article. Review.
Cited by
-
Immunothrombosis and the Role of Platelets in Venous Thromboembolic Diseases.Int J Mol Sci. 2022 Oct 29;23(21):13176. doi: 10.3390/ijms232113176. Int J Mol Sci. 2022. PMID: 36361963 Free PMC article. Review.
-
LRRC superfamily expression in stromal cells predicts the clinical prognosis and platinum resistance of ovarian cancer.BMC Med Genomics. 2023 Jan 18;16(1):10. doi: 10.1186/s12920-023-01435-9. BMC Med Genomics. 2023. PMID: 36653841 Free PMC article.
-
Mechanisms of cancer-associated thrombosis.Res Pract Thromb Haemost. 2023 Mar 15;7(3):100123. doi: 10.1016/j.rpth.2023.100123. eCollection 2023 Mar. Res Pract Thromb Haemost. 2023. PMID: 37122533 Free PMC article.
-
Exosome-transmitted podoplanin promotes tumor-associated macrophage-mediated immune tolerance in glioblastoma.CNS Neurosci Ther. 2024 Mar;30(3):e14643. doi: 10.1111/cns.14643. CNS Neurosci Ther. 2024. PMID: 38470096 Free PMC article.
-
The regulation of cancer-associated thrombosis by podoplanin.Thromb Update. 2024 Jun;15:100174. doi: 10.1016/j.tru.2024.100174. Epub 2024 Apr 17. Thromb Update. 2024. PMID: 40741180 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

