DDK/Hsk1 phosphorylates and targets fission yeast histone deacetylase Hst4 for degradation to stabilize stalled DNA replication forks
- PMID: 34608864
- PMCID: PMC8565929
- DOI: 10.7554/eLife.70787
DDK/Hsk1 phosphorylates and targets fission yeast histone deacetylase Hst4 for degradation to stabilize stalled DNA replication forks
Abstract
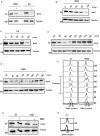
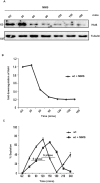
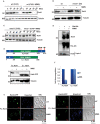
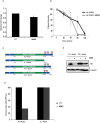
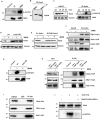
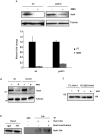
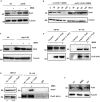
In eukaryotes, paused replication forks are prone to collapse, which leads to genomic instability, a hallmark of cancer. Dbf4-dependent kinase (DDK)/Hsk1Cdc7 is a conserved replication initiator kinase with conflicting roles in replication stress response. Here, we show that fission yeast DDK/Hsk1 phosphorylates sirtuin, Hst4 upon replication stress at C-terminal serine residues. Phosphorylation of Hst4 by DDK marks it for degradation via the ubiquitin ligase SCFpof3. Phosphorylation-defective hst4 mutant (4SA-hst4) displays defective recovery from replication stress, faulty fork restart, slow S-phase progression and decreased viability. The highly conserved fork protection complex (FPC) stabilizes stalled replication forks. We found that the recruitment of FPC components, Swi1 and Mcl1 to the chromatin is compromised in the 4SA-hst4 mutant, although whole cell levels increased. These defects are dependent upon H3K56ac and independent of intra S-phase checkpoint activation. Finally, we show conservation of H3K56ac-dependent regulation of Timeless, Tipin, and And-1 in human cells. We propose that degradation of Hst4 via DDK increases H3K56ac, changing the chromatin state in the vicinity of stalled forks facilitating recruitment and function of FPC. Overall, this study identified a crucial role of DDK and FPC in the regulation of replication stress response with implications in cancer therapeutics.
Keywords: H3K56ac; S. pombe; Swi1; chromosomes; gene expression; genome stability; replication fork; sirtuin; timeless.
© 2021, Aricthota and Haldar.
Conflict of interest statement
SA, DH No competing interests declared
Figures









Similar articles
-
Hsk1-Dfp1/Him1, the Cdc7-Dbf4 kinase in Schizosaccharomyces pombe, associates with Swi1, a component of the replication fork protection complex.J Biol Chem. 2005 Dec 30;280(52):42536-42. doi: 10.1074/jbc.M510575200. Epub 2005 Oct 31. J Biol Chem. 2005. PMID: 16263721
-
Interactions between Swi1-Swi3, Mrc1 and S phase kinase, Hsk1 may regulate cellular responses to stalled replication forks in fission yeast.Genes Cells. 2009 Jun;14(6):669-82. doi: 10.1111/j.1365-2443.2009.01300.x. Epub 2009 Apr 30. Genes Cells. 2009. PMID: 19422421 Free PMC article.
-
Fission Yeast Sirtuin Hst4 Functions in Preserving Genomic Integrity by Regulating Replisome Component Mcl1.Sci Rep. 2018 May 31;8(1):8496. doi: 10.1038/s41598-018-26476-4. Sci Rep. 2018. PMID: 29855479 Free PMC article.
-
Histones on fire: the effect of Dun1 and Mrc1 on origin firing and replication of hyper-acetylated genomes.Curr Genet. 2021 Aug;67(4):501-510. doi: 10.1007/s00294-021-01175-2. Epub 2021 Mar 14. Curr Genet. 2021. PMID: 33715066 Review.
-
Dbf4-Dependent Kinase: DDK-ated to post-initiation events in DNA replication.Cell Cycle. 2021 Nov;20(22):2348-2360. doi: 10.1080/15384101.2021.1986999. Epub 2021 Oct 18. Cell Cycle. 2021. PMID: 34662256 Free PMC article. Review.
Cited by
-
Histone acetylation dynamics in repair of DNA double-strand breaks.Front Genet. 2022 Sep 9;13:926577. doi: 10.3389/fgene.2022.926577. eCollection 2022. Front Genet. 2022. PMID: 36159966 Free PMC article. Review.
References
-
- Bianco JN, Bergoglio V, Lin Y-L, Pillaire M-J, Schmitz A-L, Gilhodes J, Lusque A, Mazières J, Lacroix-Triki M, Roumeliotis TI, Choudhary J, Moreaux J, Hoffmann J-S, Tourrière H, Pasero P. Overexpression of claspin and timeless protects cancer cells from replication stress in a checkpoint-independent manner. Nature Communications. 2019;10:910. doi: 10.1038/s41467-019-08886-8. - DOI - PMC - PubMed
-
- Blake D, Luke B, Kanellis P, Jorgensen P, Goh T, Penfold S, Breitkreutz BJ, Durocher D, Peter M, Tyers M. The f-box protein dia2 overcomes replication impedance to promote genome stability in Saccharomyces cerevisiae. Genetics. 2006;174:1709–1727. doi: 10.1534/genetics.106.057836. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

