FTSH4 and OMA1 mitochondrial proteases reduce moderate heat stress-induced protein aggregation
- PMID: 34608962
- PMCID: PMC8491029
- DOI: 10.1093/plphys/kiab296
FTSH4 and OMA1 mitochondrial proteases reduce moderate heat stress-induced protein aggregation
Abstract
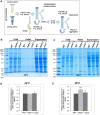
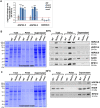
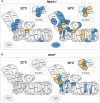
The threat of global warming makes uncovering mechanisms of plant tolerance to long-term moderate heat stress particularly important. We previously reported that Arabidopsis (Arabidopsis thaliana) plants lacking mitochondrial proteases FTSH4 or OMA1 suffer phenotypic changes under long-term stress of 30°C, while their growth at 22°C is not affected. Here we found that these morphological and developmental changes are associated with increased accumulation of insoluble mitochondrial protein aggregates that consist mainly of small heat-shock proteins (sHSPs). Greater accumulation of sHSPs in ftsh4 than oma1 corresponds with more severe phenotypic abnormalities. We showed that the proteolytic activity of FTSH4, and to a lesser extent of OMA1, as well as the chaperone function of FTSH4, is crucial for protecting mitochondrial proteins against aggregation. We demonstrated that HSP23.6 and NADH dehydrogenase subunit 9 present in aggregates are proteolytic substrates of FTSH4, and this form of HSP23.6 is also a substrate of OMA1 protease. In addition, we found that the activity of FTSH4 plays an important role during recovery from elevated to optimal temperatures. Isobaric tags for relative and absolute quantification (iTRAQ)-based proteomic analyses, along with identification of aggregation-prone proteins, implicated mitochondrial pathways affected by protein aggregation (e.g. assembly of complex I) and revealed that the mitochondrial proteomes of ftsh4 and oma1 plants are similarly adapted to long-term moderate heat stress. Overall, our data indicate that both FTSH4 and OMA1 increase the tolerance of plants to long-term moderate heat stress by reducing detergent-tolerant mitochondrial protein aggregation.
© The Author(s) 2021. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures





References
-
- Balchin D, Hayer-Hartl M, Hartl FU (2016) In vivo aspects of protein folding and quality control. Science 353: aac4354. - PubMed
-
- Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M (2003) Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem 278: 21601–21606 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

