Thymus pathology in myasthenia gravis with anti-acetylcholine receptor antibodies and concomitant Hashimoto's thyroiditis. A four-case series and literature review
- PMID: 34609410
- PMCID: PMC8597376
- DOI: 10.47162/RJME.62.1.07
Thymus pathology in myasthenia gravis with anti-acetylcholine receptor antibodies and concomitant Hashimoto's thyroiditis. A four-case series and literature review
Abstract
Objective: Identifying the morphological features of thymus in patients with myasthenia gravis (MG) with anti-acetylcholine receptor (AChR) antibodies and concomitant Hashimoto's thyroiditis (HT), which were recruited from a single surgical unit of a tertiary referral hospital located in the North-Eastern region of Romania, over a period of 11 years.
Patients, materials and methods: We retrospectively reviewed clinical, imaging, laboratory, thymic pathology, and outcome data that were obtained from medical records of patients with MG and concomitant HT, to whom a thymectomy was performed for a suspected thymic lesion. All the surgical interventions were done in the Third Clinic of Surgery, St. Spiridon Emergency County Hospital, Iaşi, Romania, for an 11 years' period, i.e., from January 1, 2000 and December 31, 2010.
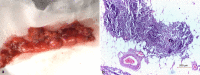
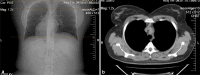
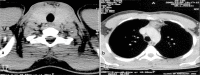
Results: Four patients (three females and one male) were included. The mean age of the patients at the time of their thymectomy was 40.25 years. Of all patients, 75% had moderate or severe MG, 100% had anti-AChR antibodies, and an electromyographic decrement greater than 25%. All patients have been diagnosed with HT in their past medical history by a full thyroid panel [high thyroid-stimulating hormone (TSH) values, low free thyroxine (fT4) values, and the presence of the anti-thyroid antibodies] and all of them have been treated with Euthyrox. Our four patients expressed different MG subtypes, each of them being associated with different thymus pathology. Thoracic computed tomography (CT) scan revealed heterogeneous mediastinal masses and established the correct diagnosis only in 25% of cases. The pathological exams also revealed a heterogeneous pattern of thymic lesions. In contrast with other studies, our patients with MG with anti-AChR antibodies and concomitant HT presented atrophic thymus more frequently (50%), but with particular morphological changes of Hassall's corpuscles. Also, 25% of cases were diagnosed with thymic lympho-follicular hyperplasia (TLFH) associated with thymic epithelial hyperplasia. In B2 thymoma, neoplastic epithelial cells expressed cytokeratin 19 (CK19) immunoreactivity, high Ki67 labeling index and strong p63 immunopositivity.
Conclusions: In our series, MG and HT occurred simultaneously, or one of them was diagnosed before the other, raising some new questions regarding the immune mechanism of these two autoimmune diseases. Due to the heterogeneous morphological changes of the thymus that we found in this study, we can hypothesize that thymus is involved in the pathogenic mechanism of MG with anti-AChR-antibodies and concomitant HT development.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures





Similar articles
-
The predictive value of the presence of different antibodies and thymus pathology to the clinical outcome in patients with generalized myasthenia gravis.Clin Neurol Neurosurg. 2013 Apr;115(4):432-7. doi: 10.1016/j.clineuro.2012.06.013. Epub 2012 Jul 6. Clin Neurol Neurosurg. 2013. PMID: 22770539
-
Pathogenesis of myasthenia gravis. Acetylcholine receptor-related antigenic determinants in tumor-free thymuses and thymic epithelial tumors.Am J Pathol. 1988 Feb;130(2):268-80. Am J Pathol. 1988. PMID: 2449082 Free PMC article.
-
Correlation between acetylcholine receptor antibody levels and thymic pathology in myasthenia gravis: a review.J Clin Neuromuscul Dis. 2013 Jun;14(4):209-17. doi: 10.1097/CND.0b013e31828a0090. J Clin Neuromuscul Dis. 2013. PMID: 23703018 Review.
-
Subclinical myasthenia gravis in thymomas.Lung Cancer. 2021 Feb;152:143-148. doi: 10.1016/j.lungcan.2020.12.010. Epub 2020 Dec 17. Lung Cancer. 2021. PMID: 33401082
-
[Thymic abnormalities in patients with myasthenia gravis].Brain Nerve. 2011 Jul;63(7):685-94. Brain Nerve. 2011. PMID: 21747138 Review. Japanese.
Cited by
-
Short histological kaleidoscope - recent findings in histology. Part II.Rom J Morphol Embryol. 2022 Apr-Jun;63(2):275-292. doi: 10.47162/RJME.63.2.01. Rom J Morphol Embryol. 2022. PMID: 36374135 Free PMC article.
-
Autoimmune thyroid disease and myasthenia gravis: a study bidirectional Mendelian randomization.Front Endocrinol (Lausanne). 2024 Feb 9;15:1310083. doi: 10.3389/fendo.2024.1310083. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38405140 Free PMC article.
-
A spatial human thymus cell atlas mapped to a continuous tissue axis.bioRxiv [Preprint]. 2023 Oct 27:2023.10.25.562925. doi: 10.1101/2023.10.25.562925. bioRxiv. 2023. Update in: Nature. 2024 Nov;635(8039):708-718. doi: 10.1038/s41586-024-07944-6. PMID: 37986877 Free PMC article. Updated. Preprint.
-
Oxidative stress in Hashimoto's thyroiditis: possible adjuvant therapies to attenuate deleterious effects.Mol Cell Biochem. 2023 Apr;478(4):949-966. doi: 10.1007/s11010-022-04564-4. Epub 2022 Sep 27. Mol Cell Biochem. 2023. PMID: 36168075 Review.
References
-
- ***. Prevalence and incidence of rare diseases: Bibliographic data. Prevalence, incidence or number of published cases listed by diseases (in alphabetical order). Orphanet Report Series, 2019, Number 1. Available at: https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseas...[ Accessed October 2019]
-
- Jaretzki A, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, Sanders DB. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55(1):16–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

