Gestational Exposure to Ultrafine Particles Reveals Sex- and Dose-Specific Changes in Offspring Birth Outcomes, Placental Morphology, and Gene Networks
- PMID: 34609516
- PMCID: PMC8633888
- DOI: 10.1093/toxsci/kfab118
Gestational Exposure to Ultrafine Particles Reveals Sex- and Dose-Specific Changes in Offspring Birth Outcomes, Placental Morphology, and Gene Networks
Abstract
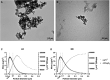
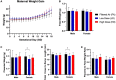
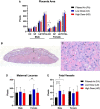
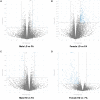
Particulate matter (PM) causes adverse developmental outcomes following prenatal exposure, but the underlying biological mechanisms remain uncertain. Here we elucidate the effects of diesel exhaust ultrafine particle (UFP) exposure during pregnancy on placental and fetal development. Time-mated C57Bl/6n mice were gestationally exposed to UFPs at a low dose (LD, 100 µg/m3) or high dose (HD, 500 µg/m3) for 6 h daily. Phenotypic effects on fetuses and placental morphology at gestational day (GD) of 18.5 were evaluated, and RNA sequencing was characterized for transcriptomic changes in placental tissue from male and female offspring. A significant decrease in average placental weights and crown to rump lengths was observed in female offspring in the LD exposure group. Gestational UFP exposure altered placental morphology in a dose- and sex-specific manner. Average female decidua areas were significantly greater in the LD and HD groups. Maternal lacunae mean areas were increased in the female LD group, whereas fetal blood vessel mean areas were significantly greater in the male LD and HD groups. RNA sequencing indicated several disturbed cellular functions related to lipid metabolism, which were most pronounced in the LD group and especially in female placental tissue. Our findings demonstrate the vulnerability of offspring exposed to UFPs during pregnancy, highlighting sex-specific effects and emphasizing the importance of mitigating PM exposure to prevent adverse health outcomes.
Keywords: air pollution; developmental and reproductive toxicology; gestational exposure; placenta development; ultrafine particulate matter.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
NRF2-Dependent Placental Effects Vary by Sex and Dose following Gestational Exposure to Ultrafine Particles.Antioxidants (Basel). 2022 Feb 10;11(2):352. doi: 10.3390/antiox11020352. Antioxidants (Basel). 2022. PMID: 35204234 Free PMC article.
-
In utero exposure to ultrafine particles promotes placental stress-induced programming of renin-angiotensin system-related elements in the offspring results in altered blood pressure in adult mice.Part Fibre Toxicol. 2019 Jan 28;16(1):7. doi: 10.1186/s12989-019-0289-1. Part Fibre Toxicol. 2019. PMID: 30691489 Free PMC article.
-
In Utero Ultrafine Particulate Exposure Yields Sex- and Dose-Specific Responses to Neonatal Respiratory Syncytial Virus Infection.Environ Sci Technol. 2022 Aug 16;56(16):11527-11535. doi: 10.1021/acs.est.2c02786. Epub 2022 Aug 4. Environ Sci Technol. 2022. PMID: 35926851 Free PMC article.
-
Air pollution and children's health-a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter.Environ Health Prev Med. 2021 Jul 12;26(1):72. doi: 10.1186/s12199-021-00995-5. Environ Health Prev Med. 2021. PMID: 34253165 Free PMC article. Review.
-
Particulate Air Pollution and Risk of Neuropsychiatric Outcomes. What We Breathe, Swallow, and Put on Our Skin Matters.Int J Environ Res Public Health. 2021 Nov 3;18(21):11568. doi: 10.3390/ijerph182111568. Int J Environ Res Public Health. 2021. PMID: 34770082 Free PMC article. Review.
Cited by
-
Maternal exposure to ultrafine particles enhances influenza infection during pregnancy.Part Fibre Toxicol. 2023 Apr 17;20(1):11. doi: 10.1186/s12989-023-00521-1. Part Fibre Toxicol. 2023. PMID: 37069680 Free PMC article.
-
Impact of PM2.5 Exposure from Wood Combustion on Reproductive Health: Implications for Fertility, Ovarian Function, and Fetal Development.Toxics. 2025 Mar 24;13(4):238. doi: 10.3390/toxics13040238. Toxics. 2025. PMID: 40278554 Free PMC article.
-
Maternal nano-titanium dioxide inhalation alters fetoplacental outcomes in a sexually dimorphic manner.Front Toxicol. 2023 Mar 6;5:1096173. doi: 10.3389/ftox.2023.1096173. eCollection 2023. Front Toxicol. 2023. PMID: 36950144 Free PMC article.
-
Integrated analysis of an in vivo model of intra-nasal exposure to instilled air pollutants reveals cell-type specific responses in the placenta.Sci Rep. 2022 May 19;12(1):8438. doi: 10.1038/s41598-022-12340-z. Sci Rep. 2022. PMID: 35589747 Free PMC article.
-
Evaluating maternal exposure to an environmental per and polyfluoroalkyl substances (PFAS) mixture during pregnancy: Adverse maternal and fetoplacental effects in a New Zealand White (NZW) rabbit model.Sci Total Environ. 2022 Sep 10;838(Pt 4):156499. doi: 10.1016/j.scitotenv.2022.156499. Epub 2022 Jun 6. Sci Total Environ. 2022. PMID: 35679923 Free PMC article.
References
-
- Åsvold B. O., Vatten L. J., Romundstad P. R., Jenum P. A., Karumanchi S. A., Eskild A. (2011). Angiogenic factors in maternal circulation and the risk of severe fetal growth restriction. Am. J. Epidemiol. 173, 630–639. - PubMed
-
- Barker D. J. (1997). Fetal nutrition and cardiovascular disease in later life. Br. Med. Bull. 53, 96–108. - PubMed
-
- Barker D. J., Larsen G., Osmond C., Thornburg K. L., Kajantie E., Eriksson J. G. (2012). The placental origins of sudden cardiac death. Int. J. Epidemiol. 41, 1394–1399. - PubMed
-
- Barker D. J., Osmond C., Forsén T. J., Thornburg K. L., Kajantie E., Eriksson J. G. (2013a). Foetal and childhood growth and asthma in adult life. Acta Paediatr. 102, 732–738. - PubMed
-
- Barker D. J., Osmond C., Thornburg K. L., Kajantie E., Eriksson J. G. (2013b). The intrauterine origins of Hodgkin's lymphoma. Cancer Epidemiol. 37, 321–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

