Effect of oral chlorhexidine de-adoption and implementation of an oral care bundle on mortality for mechanically ventilated patients in the intensive care unit (CHORAL): a multi-center stepped wedge cluster-randomized controlled trial
- PMID: 34609548
- PMCID: PMC8490143
- DOI: 10.1007/s00134-021-06475-2
Effect of oral chlorhexidine de-adoption and implementation of an oral care bundle on mortality for mechanically ventilated patients in the intensive care unit (CHORAL): a multi-center stepped wedge cluster-randomized controlled trial
Abstract
Purpose: Oral chlorhexidine is used widely for mechanically ventilated patients to prevent pneumonia, but recent studies show an association with excess mortality. We examined whether de-adoption of chlorhexidine and parallel implementation of a standardized oral care bundle reduces intensive care unit (ICU) mortality in mechanically ventilated patients.
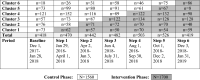
Methods: A stepped wedge cluster-randomized controlled trial with concurrent process evaluation in 6 ICUs in Toronto, Canada. Clusters were randomized to de-adopt chlorhexidine and implement a standardized oral care bundle at 2-month intervals. The primary outcome was ICU mortality. Secondary outcomes were time to infection-related ventilator-associated complications (IVACs), oral procedural pain and oral health dysfunction. An exploratory post hoc analysis examined time to extubation in survivors.
Results: A total of 3260 patients were enrolled; 1560 control, 1700 intervention. ICU mortality for the intervention and control periods were 399 (23.5%) and 330 (21.2%), respectively (adjusted odds ratio [aOR], 1.13; 95% confidence interval [CI] 0.82 to 1.54; P = 0.46). Time to IVACs (adjusted hazard ratio [aHR], 1.06; 95% CI 0.44 to 2.57; P = 0.90), time to extubation (aHR 1.03; 95% CI 0.85 to 1.23; P = 0.79) (survivors) and oral procedural pain (aOR, 0.62; 95% CI 0.34 to 1.10; P = 0.10) were similar between control and intervention periods. However, oral health dysfunction scores (- 0.96; 95% CI - 1.75 to - 0.17; P = 0.02) improved in the intervention period.
Conclusion: Among mechanically ventilated ICU patients, no benefit was observed for de-adoption of chlorhexidine and implementation of an oral care bundle on ICU mortality, IVACs, oral procedural pain, or time to extubation. The intervention may improve oral health.
Trial registration: ClinicalTrials.gov NCT03382730.
Keywords: Chlorhexidine; Critical care; De-adoption; Oral health; Randomized controlled trial; Respiration, artificial.
© 2021. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors have no conflicts of interest to report in the conduct of this research.
Figures
Comment in
-
De-adoption of chlorhexidine oral care and ICU mortality.Intensive Care Med. 2022 May;48(5):624-625. doi: 10.1007/s00134-022-06621-4. Epub 2022 Jan 17. Intensive Care Med. 2022. PMID: 35037992 No abstract available.
-
De-adoption of chlorhexidine oral care and ICU mortality. Authors' reply.Intensive Care Med. 2022 May;48(5):626-627. doi: 10.1007/s00134-022-06649-6. Epub 2022 Feb 16. Intensive Care Med. 2022. PMID: 35174399 No abstract available.
References
-
- Klompas M, Branson R, Eichenwald EC, Greene LR, Howell MD, Lee G, Magill SS, Maragakis LL, Priebe GP, Speck K, Yokoe DS, Berenholtz SM. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:915–936. doi: 10.1086/677144. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical