Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial
- PMID: 34609549
- PMCID: PMC8490137
- DOI: 10.1007/s00134-021-06507-x
Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial
Abstract
Purpose: Trials of tocilizumab in patients with severe COVID-19 pneumonia have demonstrated mixed results, and the role of tocilizumab in combination with other treatments is uncertain. Here we evaluated whether tocilizumab plus remdesivir provides greater benefit than remdesivir alone in patients with severe COVID-19 pneumonia.
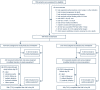
Methods: This randomized, double-blind, placebo-controlled, multicenter trial included patients hospitalized with severe COVID-19 pneumonia requiring > 6 L/min supplemental oxygen. Patients were randomly assigned (2:1 ratio) to receive tocilizumab 8 mg/kg or placebo intravenously plus ≤ 10 days of remdesivir. The primary outcome was time from randomization to hospital discharge or "ready for discharge" (defined as category 1, assessed by the investigator on a 7-category ordinal scale of clinical status) to day 28. Patients were followed for 60 days.
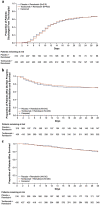
Results: Among 649 enrolled patients, 434 were randomly assigned to tocilizumab plus remdesivir and 215 to placebo plus remdesivir. 566 patients (88.2%) received corticosteroids during the trial to day 28. Median time from randomization to hospital discharge or "ready for discharge" was 14 (95% CI 12-15) days with tocilizumab plus remdesivir and 14 (95% CI 11-16) days with placebo plus remdesivir [log-rank P = 0.74; Cox proportional hazards ratio 0.97 (95% CI 0.78-1.19)]. Serious adverse events occurred in 128 (29.8%) tocilizumab plus remdesivir and 72 (33.8%) placebo plus remdesivir patients; 78 (18.2%) and 42 (19.7%) patients, respectively, died by day 28.
Conclusions: Tocilizumab plus remdesivir did not shorten time to hospital discharge or "ready for discharge" to day 28 compared with placebo plus remdesivir in patients with severe COVID-19 pneumonia.
Keywords: COVID-19; Pneumonia; Remdesivir; Tocilizumab.
© 2021. The Author(s).
Conflict of interest statement
IOR Grant from Roche/Genentech related to the submitted work and grant from Genentech and personal fees from Genentech, Boehringer, and Bristol Myers Squibb outside the submitted work. GD: Grants from Gilead Sciences, Regeneron Inc., Roche, Boehringer Ingelheim, and Edasa Biotech outside the submitted work. Gilead Medical Affairs sentinel panel and Scientific Advisory Board for Safeology Inc. RLG: Personal fees from Eli Lilly, Gilead Sciences Inc., GlaxoSmithKline, Johnson and Johnson, and Roivant Sciences Inc. and nonfinancial support from Gilead Sciences Inc. outside the submitted work. SML: Investigator fees from Roche/Genentech during the conduct of the trial. PR: Nothing to disclose. BDH: Personal fees from Kite Pharma and Novartis outside the submitted work. AWC: Nothing to disclose. JSO: Institutional funding from Roche during conduct of the trial. NAH: Grants from GlaxoSmithKline, Sanofi, AstraZeneca, Genentech, Boehringer Ingelheim, Novartis, and Gossamer Bio; personal fees from GlaxoSmithKline, Sanofi, AstraZeneca, Genentech, Novartis, Regeneron, Teva, and Amgen outside the submitted work. AS: Personal fees from Genentech, AbbVie, Pharmacyclics, Janssen, Kite Pharma, Celgene, Verastem, BeiGene, Novartis, TG Therapeutics, Seattle Genetics, Morphosys, Jazz Pharmaceuticals, and Gilead Sciences and nonfinancial support from Bristol Myers Squibb outside the submitted work. JG-D: Nothing to disclose. IG: Nothing to disclose. JC: Grant provided to institution from Roche/Genentech during conduct of the trial. Grants and personal fees from Gilead Sciences Inc. outside the submitted work. OG: Employee of Roche and shareholder of Roche Holding AG. EG: Employee of Roche. NL-K: Employee of Roche/Genentech. LT: Employee of Roche/Genentech and has an unpublished patent pending, “Tocilizumab and Remdesivir Combination Therapy for COVID-19 Pneumonia.” KT: Former employee of Roche/Genentech and owns stock in Roche/Genentech. HC: Employee of Gilead Sciences Inc. DB: Former employee of and holds stock in Gilead Sciences Inc. JKO: Employee of Roche/Genentech.
Figures
References
-
- World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed July 6, 2021
-
- World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance 13 March 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management.... Accessed July 6, 2021
-
- World Health Organization. Coronavirus disease (COVID-2019) weekly epidemiological update and weekly operational update. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio.... Accessed July 6, 2021
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

