Progress of CD47 immune checkpoint blockade agents in anticancer therapy: a hematotoxic perspective
- PMID: 34609596
- PMCID: PMC11800891
- DOI: 10.1007/s00432-021-03815-z
Progress of CD47 immune checkpoint blockade agents in anticancer therapy: a hematotoxic perspective
Abstract
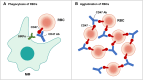
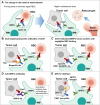
CD47, a transmembrane protein, acts as a "do not eat me" signal that is overexpressed in many tumor cell types, thereby forming a signaling axis with its ligand signal regulatory protein alpha (SIRPα) and enabling the tumor cells to escape from macrophage-mediated phagocytosis. Several clinical trials with CD47 targeting agents are underway and have achieved impressive results preliminarily. However, hematotoxicity (particularly anemia) has emerged as the most common side effect that cannot be neglected. In the development of CD47 targeting agents, various methods have been used to mitigate this toxicity. In this review, we summarized five strategies used to alleviate CD47 blockade-induced hematotoxicity, as follows: change in the mode of administration; dual targeting bispecific antibodies of CD47; CD47 antibodies/SIRPα fusion proteins with negligible red blood cell binding; anti-SIRPα antibodies; and glutaminyl-peptide cyclotransferase like inhibitors. With these strategies, the development of CD47 targeting agents can be improved.
Keywords: Anemia; CD47/SIRPα; Immunotherapy; Macrophages; Phagocytosis checkpoint.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declared that there is no conflict of interest.
Figures
Similar articles
-
Novel immunotherapy for gastric cancer: targeting the CD47-SIRPα axis.Cancer Metastasis Rev. 2025 May 29;44(2):52. doi: 10.1007/s10555-025-10269-z. Cancer Metastasis Rev. 2025. PMID: 40437298 Review.
-
A human antibody specific for SIRPα reprograms macrophages and promotes antibody mediated anti-cancer activity.PLoS One. 2025 May 23;20(5):e0321169. doi: 10.1371/journal.pone.0321169. eCollection 2025. PLoS One. 2025. PMID: 40408355 Free PMC article.
-
Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis.Front Immunol. 2020 Jan 28;11:18. doi: 10.3389/fimmu.2020.00018. eCollection 2020. Front Immunol. 2020. PMID: 32082311 Free PMC article. Review.
-
B7-H3 and CD47 co-expression in gastric cancer is a predictor of poor prognosis and potential targets for future dual-targeting immunotherapy.J Cancer Res Clin Oncol. 2023 Dec;149(18):16609-16621. doi: 10.1007/s00432-023-05408-4. Epub 2023 Sep 16. J Cancer Res Clin Oncol. 2023. PMID: 37715830 Free PMC article.
-
Functional characterization of the selective pan-allele anti-SIRPα antibody ADU-1805 that blocks the SIRPα-CD47 innate immune checkpoint.J Immunother Cancer. 2019 Dec 4;7(1):340. doi: 10.1186/s40425-019-0772-0. J Immunother Cancer. 2019. PMID: 31801627 Free PMC article.
Cited by
-
Building on the backbone of CD47-based therapy in cancer: Combination strategies, mechanisms, and future perspectives.Acta Pharm Sin B. 2023 Apr;13(4):1467-1487. doi: 10.1016/j.apsb.2022.12.016. Epub 2022 Dec 26. Acta Pharm Sin B. 2023. PMID: 37139405 Free PMC article. Review.
-
CD200R1-CD200 checkpoint inhibits phagocytosis differently from SIRPα-CD47 to suppress tumor growth.Nat Commun. 2025 Jun 3;16(1):5145. doi: 10.1038/s41467-025-60456-3. Nat Commun. 2025. PMID: 40461553 Free PMC article.
-
The advances in targeting CD47/SIRPα "do not eat me" axis and their ongoing challenges as an anticancer therapy.Oncotarget. 2024 Jul 10;15:462-465. doi: 10.18632/oncotarget.28607. Oncotarget. 2024. PMID: 38985136 Free PMC article. No abstract available.
-
Antitumor strategies targeting macrophages: the importance of considering the differences in differentiation/polarization processes between human and mouse macrophages.J Immunother Cancer. 2022 Oct;10(10):e005560. doi: 10.1136/jitc-2022-005560. J Immunother Cancer. 2022. PMID: 36270732 Free PMC article. Review.
-
Novel Phenylpiperidine Derivatives as QPCT and QPCTL Inhibitors for Treating Cancer or Fibrotic Diseases.ACS Med Chem Lett. 2024 Oct 30;15(11):1826-1827. doi: 10.1021/acsmedchemlett.4c00495. eCollection 2024 Nov 14. ACS Med Chem Lett. 2024. PMID: 39563800
References
-
- Agoram B, Wang B, Sikic BI, Lakhani NJ, Patnaik A, Liu J et al (2018) Pharmacokinetics of Hu5F9-G4, a first-in-class anti-CD47 antibody, in patients with solid tumors and lymphomas. J Clin Oncol 36(15_suppl):2525
-
- Ansell S, Chen RW, Flinn IW, Maris MB, O’Connor OA, Johnson LD et al (2016) A phase 1 study of TTI-621, a novel immune checkpoint inhibitor targeting CD47, in patients with relapsed or refractory hematologic malignancies. Blood 128(22):1812
-
- Ansell SM, Flinn IW, Maris MB, O’Connor OA, Lesokhin A, Advani AS et al (2017) TTI-621 (SIRPαFc), an immune checkpoint inhibitor blocking the CD47 “do not eat” signal, induces objective responses in patients with advanced, relapsed/refractory diffuse large B-cell lymphoma (DLBCL). Blood 130(Supplement 1):4116
-
- Ansell SM, Maris MB, Lesokhin AM, Chen RW, Flinn IW, Sawas A et al (2021) Phase I study of the CD47 blocker TTI-621 in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res 27(8):2190–2199 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

