Palladium-Catalyzed, Enantioselective Desymmetrization of N-Acylaziridines with Indoles
- PMID: 34609884
- PMCID: PMC9022218
- DOI: 10.1021/acs.orglett.1c02914
Palladium-Catalyzed, Enantioselective Desymmetrization of N-Acylaziridines with Indoles
Abstract
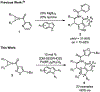
Ring opening reactions of meso-aziridines generate chiral amine derivatives where the control of stereochemistry is possible through enantioselective catalysis. We report the use of a diphosphine-palladium(II) catalyst for the highly enantioselective desymmetrization of N-acylaziridines with indoles. The β-tryptamine products are isolated in moderate to high yield across a range of indole and aziridine substitution patterns. The synthetic utility of β-tryptamine products is demonstrated by conversion to the brominated pyrroloindoline derivative.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
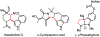


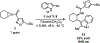

References
-
- Royer J; Husson HP Asymmetric Synthesis of Nitrogen Heterocycles; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2009.
- Nugent TC Chiral Amine Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2010.
- Mailyan AK; Eickhoff JA; Minakova AS; Gu ZH; Lu P; Zakarian A Cutting-Edge and Time-Honored Strategies for Stereoselective Construction of C–N Bonds in Total Synthesis. Chem. Rev 2016, 116, 4441–4557. - PubMed
-
- Dahanukar VH; Zavialov IA Aziridines and Aziridinium Ions in the Practical Synthesis of Pharmaceutical Intermediates - A Perspective. Curr. Opin. Drug Dis. Dev 2002, 5, 918–927. - PubMed
- Hu XE Nucleophilic Ring Opening of Aziridines. Tetrahedron 2004, 60, 2701–2743.
- Krake SH; Bergmeier SC Inter- and Intramolecular Reactions of Epoxides and Aziridines with p-Nucleophiles. Tetrahedron 2010, 66, 7337–7360.
- Ghorai MK; Bhattacharyya A; Das S; Chauhan N Ring Expansions of Activated Aziridines and Azetidines. Top. Heterocycl. Chem 2015, 41, 49–142.
- Singh G Advances in Synthesis and Chemistry of Aziridines. In Advances in Heterocyclic Chemistry; Scriven EFV, Ramsden CA, Eds.; Elsevier, Inc., 2019; Vol. 129, pp 245–335.
-
- Schneider C Catalytic, Enantioselective Ring Opening of Aziridines. Angew. Chem., Int. Ed 2009, 48, 2082–2084. - PubMed
- Wang P-A Organocatalyzed Enantioselective Desymmetrization of Aziridines and Epoxides. Beilstein J. Org. Chem 2013, 9, 1677–1695. - PMC - PubMed
- Wang Z; Hong WX; Sun J Organocatalytic Enantioselective Desymmetrization of meso-Aziridines and Prochiral Azetidines. Curr. Org. Chem 2016, 20, 1851–1861.
- Akhtar R; Naqvi SAR; Zahoor AF; Saleem S Nucleophilic Ring Opening Reactions of Aziridines. Mol. Diversity 2018, 22, 447–501. - PubMed
-
- Müller P; Nury P Copper-Catalyzed Desymmetrization of N-Sulfonylaziridines with Methylmagnesium Halides. Org. Lett 1999, 1, 439–441.
- Müller P; Nury P Desymmetrization of meso-NSulfonylaziridines with Chiral Nonracemic Nucleophiles and Bases. Helv. Chim. Acta 2001, 84, 662–677.
- Xu YJ; Lin LQ; Kanai M; Matsunaga S; Shibasaki M Catalytic Asymmetric Ring-Opening of meso-Aziridines with Malonates under Heterodinuclear Rare Earth Metal Schiff Base Catalysis. J. Am. Chem. Soc 2011, 133, 5791–5793. - PubMed
- Li D; Wang L; Yang D; Zhang B; Wang R Catalytic Desymmetrization of meso-Aziridines with Benzofuran2(3H)-Ones Employing a Simple In Situ-Generated Magnesium Catalyst. ACS Catal. 2015, 5, 7432–7436.
- Wang L; Yang D; Li D; Wang R Catalytic Enantioselective Ring-Opening and Ring-Closing Reactions of 3-Isothiocyanato Oxindoles and N-(2-Picolinoyl)aziridines. Org. Lett 2015, 17, 3004–3007. - PubMed
- Yang D; Wang L; Han F; Li D; Zhao D; Wang R Intermolecular Enantioselective Dearomatization Reaction of β-Naphthol Using meso-Aziridine: A Bifunctional In Situ Generated Magnesium Catalyst. Angew. Chem., Int. Ed 2015, 54, 2185–2189. - PubMed
- Li D; Wang Y; Wang L; Wang J; Wang P; Wang K; Lin L; Liu D; Jiang X; Yang D Simple Magnesium Catalyst Mediated γ-Butyrolactams in Desymmetrization of meso-Aziridines. Chem. Commun 2016, 52, 9640–9643. - PubMed
- Wang L; Li D; Yang D; Wang K; Wang J; Wang P; Su W; Wang R Catalytic Asymmetric Ring-Opening Reactions of Aziridines with 3-Aryl-Oxindoles. Chem. - Asian J 2016, 11, 691–695. - PubMed
- Shiomi N; Kuroda M; Nakamura S Desymmetrization of Aziridine with Malononitrile using Cinchona Alkaloid Amide/Zinc(II) Catalysts. Chem. Commun 2017, 53, 1817–1820. - PubMed
- Wang L; Yang D; Li D; Zhu H; Wang P; Liu X; Bai L; Wang R Diversiform Reactivity of Naphthols in Asymmetric Dearomatization or O-Alkylation Reactions with Aziridines. Adv. Synth. Catal 2018, 360, 4491–4496.
- Li D; Wang L; Zhu H; Bai L; Yang Y; Zhang M; Yang D; Wang R Catalytic Asymmetric Reactions of α-Isocyanoacetates and meso-Aziridines Mediated by an in-Situ-Generated Magnesium Catalytic Method. Org. Lett 2019, 21, 4717–4720. - PubMed
- Li X; Xiong Q; Guan M; Dong S; Liu X; Feng X Divergent Synthesis of Enantioenriched β-Functional Amines via Desymmetrization of meso-Aziridines with Isocyanides. Org. Lett 2019, 21, 6096–6101. - PubMed
-
- Yang D; Wang L; Han F; Li D; Zhao D; Cao Y; Ma Y; Kong W; Sun Q; Wang R Highly Enantioselective Ring-Opening Reactions of Aziridines with Indole and Its Application in the Building of C-3-Halogenated Pyrroloindolines. Chem. - Eur. J 2014, 20, 16478–16483. - PubMed
- Wang L; Yang D; Han F; Li D; Zhao D; Wang R Catalytic Asymmetric Construction of Pyrroloindolines via an in Situ Generated Magnesium Catalyst. Org. Lett 2015, 17, 176–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

