Spatial bias in cAMP generation determines biological responses to PTH type 1 receptor activation
- PMID: 34609896
- PMCID: PMC8682804
- DOI: 10.1126/scisignal.abc5944
Spatial bias in cAMP generation determines biological responses to PTH type 1 receptor activation
Erratum in
-
Erratum for the Research Article "Spatial bias in cAMP generation determines biological responses to PTH type 1 receptor activation" by A. D White, K. A. Peña, L. J. Clark, C. Santa Maria, S. Liu, F. G. Jean-Alphonse, J. Y. Lee, S. Lei, Z. Cheng, C.-L. Tu, F. Fang, N. Szeto, T. J. Gardella, K. Xiao, S. H. Gellman, I. Bahar, I. Sutkeviciute, W. Chang, J.-P. Vilardaga.Sci Signal. 2021 Oct 26;14(706):eabm8482. doi: 10.1126/scisignal.abm8482. Epub 2021 Oct 26. Sci Signal. 2021. PMID: 34699251 No abstract available.
Abstract
The parathyroid hormone (PTH) type 1 receptor (PTHR) is a class B G protein–coupled receptor (GPCR) that regulates mineral ion, vitamin D, and bone homeostasis. Activation of the PTHR by PTH induces both transient cell surface and sustained endosomal cAMP production. To address whether the spatial (location) or temporal (duration) dimension of PTHR-induced cAMP encodes distinct biological outcomes, we engineered a biased PTHR ligand (PTH7d) that elicits cAMP production at the plasma membrane but not at endosomes. PTH7d stabilized a unique active PTHR conformation that mediated sustained cAMP signaling at the plasma membrane due to impaired β-arrestin coupling to the receptor. Experiments in cells and mice revealed that sustained cAMP production by cell surface PTHR failed to mimic the pharmacological effects of sustained endosomal cAMP production on the abundance of the rate-limiting hydroxylase catalyzing the formation of active vitamin D, as well as increases in circulating active vitamin D and Ca2+ and in bone formation in mice. Thus, similar amounts of cAMP generated by PTHR for similar lengths of time in different cellular locations, plasma membrane and endosomes, mediate distinct physiological responses. These results unveil subcellular signaling location as a means to achieve specificity in PTHR-mediated biological outcomes and raise the prospect of rational drug design based upon spatiotemporal manipulation of GPCR signaling.
Conflict of interest statement
Figures
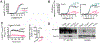
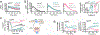
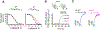

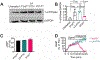

References
-
- Fredriksson R, Schioth HB, The repertoire of G-protein-coupled receptors in fully sequenced genomes. Molecular pharmacology 67, 1414–1425 (2005). - PubMed
-
- Galandrin S, Oligny-Longpre G, Bouvier M, The evasive nature of drug efficacy: implications for drug discovery. Trends in pharmacological sciences 28, 423–430 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AR066262/AR/NIAMS NIH HHS/United States
- R01 DK121656/DK/NIDDK NIH HHS/United States
- R01 GM056414/GM/NIGMS NIH HHS/United States
- P41 GM103712/GM/NIGMS NIH HHS/United States
- R01 DK116780/DK/NIDDK NIH HHS/United States
- IK6 BX004835/BX/BLRD VA/United States
- P01 DK011794/DK/NIDDK NIH HHS/United States
- I01 BX005851/BX/BLRD VA/United States
- T32 DE007306/DE/NIDCR NIH HHS/United States
- R21 AG070721/AG/NIA NIH HHS/United States
- R01 AR067291/AR/NIAMS NIH HHS/United States
- R01 GM139297/GM/NIGMS NIH HHS/United States
- I01 BX003453/BX/BLRD VA/United States
- T32 CA009120/CA/NCI NIH HHS/United States
- F31 AR074843/AR/NIAMS NIH HHS/United States
- R01 DK122259/DK/NIDDK NIH HHS/United States
- R01 DK111427/DK/NIDDK NIH HHS/United States
- P30 AR066261/AR/NIAMS NIH HHS/United States
- T32 GM088119/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

