Usability of an At-Home Anterior Nares SARS-CoV-2 RT-PCR Sample Collection Kit: Human Factors Feasibility Study
- PMID: 34609947
- PMCID: PMC8673714
- DOI: 10.2196/29234
Usability of an At-Home Anterior Nares SARS-CoV-2 RT-PCR Sample Collection Kit: Human Factors Feasibility Study
Abstract
Background: Readily available testing for SARS-CoV-2 is necessary to mitigate COVID-19 disease outbreaks. At-home collection kits, in which samples are self-collected without requiring a laboratory or clinic visit and sent to an external laboratory for testing, can provide convenient testing to those with barriers to access. They can prevent unnecessary exposure between patient and clinical staff, increase access for patients with disabilities or remote workers, and decrease burdens on health care resources, such as provider time and personal protective equipment. Exact Sciences developed an at-home collection kit for samples to be tested to detect SARS-CoV-2 that includes an Instructions for Use (IFU) document, which guides people without prior experience on collecting a nasal swab sample. Demonstrating successful sample collection and usability is critical to ensure that these samples meet the same high-quality sample collection standards as samples collected in clinics.
Objective: The aim of this study was to determine the usability of a SARS-CoV-2 at-home nasal swab sample collection kit.
Methods: A human factors usability study was conducted with 30 subjects without prior medical, laboratory, or health care training and without COVID-19 sample self-collection experience. Subjects were observed while they followed the IFU for the at-home sample collection portion of the SARS-CoV-2 test in a setting that simulated a home environment. IFU usability was further evaluated by requiring the subjects to complete a survey, answer comprehension questions, provide written feedback, and respond to questions from the observer about problems during use.
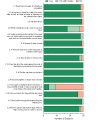
Results: All 30 subjects successfully completed the sample collection process, and all 30 samples were determined by reverse transcription-polymerase chain reaction (RT-PCR) testing to meet quality standards for SARS-CoV-2 testing. The subjects' written feedback and comments revealed several recommendations to improve the IFU.
Conclusions: The study demonstrated the overall usability of an at-home SARS-CoV-2 collection kit. Various feedback mechanisms provided opportunities to improve the wording and graphics for some critical tasks, including placing the label correctly on the tube. A modified IFU was prepared based on study outcomes.
Keywords: COVID-19; COVID-19 testing; SARS-CoV-2; at-home collection kit; feasibility studies; self-collection; usability study.
©Laura E Strong, Irene Middendorf, Michelle Turner, David K Edwards V, Varun Sama, Joshua Mou, K Colleen Adams. Originally published in JMIR Human Factors (https://humanfactors.jmir.org), 14.12.2021.
Conflict of interest statement
Conflicts of Interest: MT, VS, DKEV, JM, and KCA are employees of Exact Sciences Corporation. LES and IM were employees of Exact Sciences Corporation when this study was conducted.
Figures
References
-
- WHO Coronavirus (COVID-19) Dashboard. World Health Organization. 2021. [2021-09-02]. https://covid19.who.int/
-
- Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, COVID-19 Systematic Urgent Review Group Effort (SURGE) Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet. 2020 Jun 27;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(20)31142-9 S0140-6736(20)31142-9 - DOI - PMC - PubMed
-
- Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021 Feb;21(2):e26–e35. doi: 10.1016/S1473-3099(20)30773-8. http://europepmc.org/abstract/MED/33125914 S1473-3099(20)30773-8 - DOI - PMC - PubMed
-
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. http://europepmc.org/abstract/MED/33301246 - DOI - PMC - PubMed
-
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021 Feb 04;384(5):403–416. doi: 10.1056/NEJMoa2035389. http://europepmc.org/abstract/MED/33378609 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous