Early reduction in PD-L1 expression predicts faster treatment response in human cutaneous leishmaniasis
- PMID: 34609968
- PMCID: PMC8592550
- DOI: 10.1172/JCI142765
Early reduction in PD-L1 expression predicts faster treatment response in human cutaneous leishmaniasis
Abstract
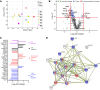
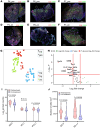
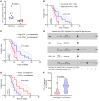
Cutaneous leishmaniasis (CL) is caused by Leishmania donovani in Sri Lanka. Pentavalent antimonials (e.g., sodium stibogluconate [SSG]) remain first-line drugs for CL with no new effective treatments emerging. We studied whole blood and lesion transcriptomes from Sri Lankan patients with CL at presentation and during SSG treatment. From lesions but not whole blood, we identified differential expression of immune-related genes, including immune checkpoint molecules, after onset of treatment. Using spatial profiling and RNA-FISH, we confirmed reduced expression of programmed death-ligand 1 (PD-L1) and indoleamine 2,3-dioxygenase 1 (IDO1) proteins on treatment in lesions of a second validation cohort and further demonstrated significantly higher expression of these checkpoint molecules on parasite-infected compared with noninfected lesional CD68+ monocytes and macrophages. Crucially, early reduction in PD-L1 but not IDO1 expression was predictive of rate of clinical cure (HR = 4.88) and occurred in parallel with reduction in parasite load. Our data support a model whereby the initial anti-leishmanial activity of antimonial drugs alleviates checkpoint inhibition on T cells, facilitating immune-drug synergism and clinical cure. Our findings demonstrate that PD-L1 expression can be used as a predictor of rapidity of clinical response to SSG treatment in Sri Lanka and support further evaluation of PD-L1 as a host-directed therapeutic in leishmaniasis.
Keywords: Immunology; Immunotherapy; Infectious disease; Molecular pathology; Parasitology.
Conflict of interest statement
Figures

Similar articles
-
IL-32-producing CD8+ memory T cells define immunoregulatory niches in human cutaneous leishmaniasis.J Clin Invest. 2025 May 15;135(10):e182040. doi: 10.1172/JCI182040. eCollection 2025 May 15. J Clin Invest. 2025. PMID: 40371647 Free PMC article.
-
Treatment failure to sodium stibogluconate in cutaneous leishmaniasis: A challenge to infection control and disease elimination.PLoS One. 2021 Oct 22;16(10):e0259009. doi: 10.1371/journal.pone.0259009. eCollection 2021. PLoS One. 2021. PMID: 34679130 Free PMC article.
-
Programmed Cell Death Ligand (PD-L)-1 Contributes to the Regulation of CD4+ T Effector and Regulatory T Cells in Cutaneous Leishmaniasis.Front Immunol. 2020 Oct 22;11:574491. doi: 10.3389/fimmu.2020.574491. eCollection 2020. Front Immunol. 2020. PMID: 33193363 Free PMC article.
-
Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies.Am J Trop Med Hyg. 1992 Mar;46(3):296-306. doi: 10.4269/ajtmh.1992.46.296. Am J Trop Med Hyg. 1992. PMID: 1313656 Review.
-
Management of cutaneous leishmaniasis.Curr Opin Infect Dis. 2001 Apr;14(2):151-4. doi: 10.1097/00001432-200104000-00007. Curr Opin Infect Dis. 2001. PMID: 11979125 Review.
Cited by
-
Evaluation of Less Invasive Sampling Tools for the Diagnosis of Cutaneous Leishmaniasis.Open Forum Infect Dis. 2024 Feb 28;11(4):ofae113. doi: 10.1093/ofid/ofae113. eCollection 2024 Apr. Open Forum Infect Dis. 2024. PMID: 38560600 Free PMC article.
-
A randomized, double-blind phase 2b trial to evaluate efficacy of ChAd63-KH for treatment of post kala-azar dermal leishmaniasis.Mol Ther Methods Clin Dev. 2024 Jul 30;32(3):101310. doi: 10.1016/j.omtm.2024.101310. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39253357 Free PMC article.
-
Characterisation of tumor microenvironment and prevalence of CD274/PD-L1 genetic alterations difference in colorectal Cancer.BMC Cancer. 2023 Mar 9;23(1):221. doi: 10.1186/s12885-023-10610-1. BMC Cancer. 2023. PMID: 36894899 Free PMC article.
-
Visceral Leishmaniasis and the Skin: Dermal Parasite Transmission to Sand Flies.Pathogens. 2022 May 24;11(6):610. doi: 10.3390/pathogens11060610. Pathogens. 2022. PMID: 35745464 Free PMC article. Review.
-
Excretory/secretory proteins inhibit host immune responses by downregulating the TLR4/NF-κB/MAPKs signaling pathway: A possible mechanism of immune evasion in parasitic nematode Haemonchus contortus.Front Immunol. 2022 Sep 27;13:1013159. doi: 10.3389/fimmu.2022.1013159. eCollection 2022. Front Immunol. 2022. PMID: 36238295 Free PMC article.
References
-
- Drugs for Neglected Diseases Initiative. Cutaneous leishmaniasis. https://dndi.org/diseases/cutaneous-leishmaniasis/ Accessed October 4, 2021.
-
- Athukorale DN, et al. Locally acquired cutaneous leishmaniasis in Sri Lanka. J Trop Med Hyg. 1992;95(6):432–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

