Venetoclax and azacitidine compared with induction chemotherapy for newly diagnosed patients with acute myeloid leukemia
- PMID: 34610123
- PMCID: PMC8714726
- DOI: 10.1182/bloodadvances.2021005538
Venetoclax and azacitidine compared with induction chemotherapy for newly diagnosed patients with acute myeloid leukemia
Abstract
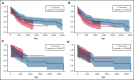
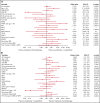
Venetoclax (ven) plus azacitidine (aza) is the standard of care for patients with newly diagnosed acute myeloid leukemia (AML) who are not candidates for intensive chemotherapy (IC). Some patients who are IC candidates instead receive ven/aza. We retrospectively analyzed patients with newly diagnosed AML who received ven/aza (n = 143) or IC (n = 149) to compare outcomes, seek variables that could predict response to 1 therapy or the other, and ascertain whether treatment recommendations could be refined. The response rates were 76.9% for ven/aza and 70.5% for IC. The median overall survival (OS) was 884 days for IC compared with 483 days for ven/aza (P = .0020). A propensity-matched cohort was used to compare outcomes in the setting of equivalent baseline variables, and when matched for age, biological risk, and transplantation, the median OS was 705 days for IC compared with not reached for ven/aza (P = .0667). Variables that favored response to ven/aza over IC included older age, secondary AML, and RUNX1 mutations. AML M5 favored response to IC over ven/aza. In the propensity-matched cohort analyzing OS, older age, adverse risk, and RUNX1 mutations favored ven/aza over IC, whereas intermediate risk favored IC over ven/aza. In conclusion, patients receiving IC have improved OS compared with those receiving ven/aza. However, in a propensity-matched cohort of patients with equivalent baseline factors, there was a trend toward favorable OS for ven/aza. Specific variables, such as RUNX1 mutations, reported here for the first time, can be identified that favor ven/aza or IC, helping to guide treatment decisions for patients who may be eligible candidates for either therapy.
© 2021 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures


References
-
- Estey EH. Acute myeloid leukemia: 2021 update on risk-stratification and management. Am J Hematol. 2020;95(11):1368-1398. - PubMed
-
- DiNardo CD, Jonas BA, Pullarkat V, et al. . Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. - PubMed
-
- DiNardo CD, Pratz KW, Letai A, et al. . Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216-228. - PubMed
-
- Pollyea DA, Pratz K, Letai A, et al. . Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol. 2021;96(2):208-217. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

