Rapid preparation of nanodiscs for biophysical studies
- PMID: 34610337
- PMCID: PMC8596956
- DOI: 10.1016/j.abb.2021.109051
Rapid preparation of nanodiscs for biophysical studies
Erratum in
-
Corrigendum to "Rapid preparation of nanodiscs for biophysical studies" [Arch. Biochem. Biophys. 712 (2021) 109051].Arch Biochem Biophys. 2021 Dec 15;714:109073. doi: 10.1016/j.abb.2021.109073. Epub 2021 Oct 30. Arch Biochem Biophys. 2021. PMID: 34756727 No abstract available.
Abstract
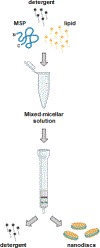
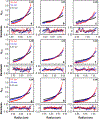
Nanodiscs, which are disc-shaped entities that contain a central lipid bilayer encased by an annulus of amphipathic helices, have emerged as a leading native-like membrane mimic. The current approach for the formation of nanodiscs involves the creation of a mixed-micellar solution containing membrane scaffold protein, lipid, and detergent followed by a time consuming process (3-12 h) of dialysis and/or incubation with sorptive beads to remove the detergent molecules from the sample. In contrast, the methodology described herein provides a facile and rapid procedure for the preparation of nanodiscs in a matter of minutes (<15 min) using Sephadex® G-25 resin to remove the detergent from the sample. A panoply of biophysical techniques including analytical ultracentrifugation, dynamic light scattering, gel filtration chromatography, circular dichroism spectroscopy, and cryogenic electron microscopy were employed to unequivocally confirm that aggregates formed by this method are indeed nanodiscs. We believe that this method will be attractive for time-sensitive and high-throughput experiments.
Keywords: Analytical ultracentrifugation; Circular dichroism spectroscopy; Cryogenic electron microscopy; Dynamic light scattering; Nanodiscs; Reconstitution.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interests.
The authors declare that there are no competing conflicts of interest.
Figures



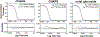



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

