Acropetal and basipetal cardenolide transport in Erysimum cheiranthoides (wormseed wallflower)
- PMID: 34610557
- PMCID: PMC8655687
- DOI: 10.1016/j.phytochem.2021.112965
Acropetal and basipetal cardenolide transport in Erysimum cheiranthoides (wormseed wallflower)
Abstract
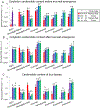
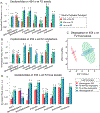
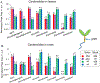
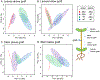
Plant specialized metabolites are often subject to within-plant transport and have tissue-specific distribution patterns. Among plants in the Brassicaceae, the genus Erysimum is unique in producing not only glucosinolates but also cardenolides. Ten cardenolides were detected with varying abundance in different tissues of Erysimum cheiranthoides L (Brassicaceae; wormseed wallflower). As is predicted by the optimal defense theory, cardenolides were most abundant in young leaves and reproductive tissues. The lowest concentrations were observed in senescing leaves and roots. Crosses between wildtype E. cheiranthoides and a mutant line with an altered cardenolide profile showed that the seed cardenolide phenotype is determined entirely by the maternal genotype. Prior to the development of the first true leaves, seedling cotyledons also had the maternal cardenolide profile. Hypocotyl grafting experiments showed that the root cardenolide profile is determined entirely by the aboveground plant genotype. In further grafting experiments, there was no evidence of cardenolide transport into the leaves, but a mixed cardenolide profile was observed in the stems and inflorescences of plants that had been grafted at vegetative and flowering growth stages, respectively. Together, these results indicate that E. cheiranthoides leaves are a site of cardenolide biosynthesis.
Keywords: Brassicaceae; Cardenolide; Cardiac glycoside; Crosses; Erysimum cheiranthoides; Grafting; Transport; Wormseed wallflower.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Alfermann AW, Boy HM, Döller PC, Hagedorn W, Heins M, Wahl J, Reinhard E, 1977. Biotransformation of Cardiac Glycosides by Plant Cell Cultures, in: Barz W, Reinhard E, Zenk MH (Eds.), Plant Tissue Culture and Its Bio-Technological Application, Proceedings in Life Sciences. Springer, Berlin, Heidelberg, pp. 125–141. 10.1007/978-3-642-66646-9_11 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

